��Ŀ����
����Ŀ���������Ƭ�Ķ�Ӱ�����У�δ�ع���廯��(AgBr)�������������(Na2S2O3)�ܽ⣬��Ӧ����Na3[Ag(S2O3)2]���ڷ϶�ӰҺ�м���Na2SʹNa3[Ag(S2O3)2]�е���ת��ΪAg2S����ʹ��ӰҺ��������Ag2S�ڸ�����ת��ΪAg���ʹﵽ�˻�������Ŀ�ġ�
(1)ͭ����������Ԫ�����ڱ���Ϊ���ɽ���Ԫ�أ����л�̬��ԭ�ӵļ۵����Ų�ʽΪ______��
(2)Na��O��S�����Ӱ뾶�ɴ�С��˳��Ϊ___________���ü����ӷ��ű�ʾ���Ӱ뾶����
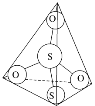
(3)S2O32-���ӽṹ��ͼ1��ʾ����������ԭ�ӵ��ӻ��������Ϊ________��
(4)Na3[Ag(S2O3)2]�д��ڵ���������_____________��
A�����Ӽ� B�����ۼ� C�����»��� D�������� E.��λ��
(5)�ڿ���������Ag2S����Ag��SO2��SO2���ӿռ乹��Ϊ____________________����SO3��ȣ�____________�ļ��Ǹ���ԭ����________________________________��

(6)��ͼ�ǽ����⻯�ﴢ����ϣ��侧����ͼ��ʾ���仯ѧʽΪ____________________����֪�þ�����ܶ�Ϊ��g/cm3����þ��������Ϊ_________ cm3���ú��ѡ�NA�Ĵ���ʽ��ʾ����
���𰸡�3d54s1 S2����O2����Na�� sp3 ABE V�� SO3 SO2��SO3����ԭ�Ӿ�Ϊsp2�ӻ���SO2����һ�Թµ��Ӷԣ����������ų����ø��� MgH2 ![]()
��������
��1����̬ͭԭ�Ӻ�����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ����ݹ���ԭ����д��̬Cuԭ�ӵļ۵����Ų�ʽ��
��2�����Ӻ�����Ӳ���Խ�࣬�����Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С��
��3��S2O32-���ӽṹ��������ԭ�ӵļ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�����Sԭ�ӵ��ӻ�������ͣ�
��4��Na3[Ag��S2O3��2]����������֮��������Ӽ���Ag���Ӻ����������λ����S-Oԭ��֮����ڼ��Թ��ۼ���
��5�����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹�ͣ�SO3�м۵��Ӷ�Ϊ3��û�й¶Ե��ӣ���SO2�м۵�����Ϊ3���¶Ե�����Ϊ1��
��6�����þ�̯����ѧʽ������![]() �����������⣻
�����������⣻
����1����̬ͭԭ�Ӻ�����29�����ӣ���3d��4s�ܼ��ϵĵ���Ϊ��۵��ӣ����ݹ���ԭ����д��̬Cuԭ�ӵļ۵����Ų�ʽΪ3d104s1��
�ʴ�Ϊ��3d104s1��
��2�����Ӻ�����Ӳ���Խ�࣬�����Ӱ뾶Խ���Ӳ�ṹ��ͬ�����ӣ����Ӱ뾶����ԭ�������������С�����Ӻ�����Ӳ���S2-��࣬O2-��Na+������Ӳ�����ͬ��ԭ������O��Na�������Ӱ뾶�Ӵ�С˳����S2-��O2-��Na+��
�ʴ�Ϊ��S2-��O2-��Na+��
��3��S2O32-���ӽṹ��������ԭ�ӵļ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�����Sԭ�ӵ��ӻ��������Ϊsp3��
�ʴ�Ϊ��sp3��
��4��Na3[Ag��S2O3��2]����������֮��������Ӽ���Ag���Ӻ����������λ����S-Oԭ��֮����ڼ��Թ��ۼ������Ըýṹ�д������Ӽ�����λ�����ۼ���
��ѡABE��
��5��SO2��������ԭ�ӵļ۲���Ӷ���=![]() =3�Һ���1���µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹��ΪV�Σ�SO3�м۵��Ӷ�Ϊ3��û�й¶Ե��ӣ���SO2�м۵�����Ϊ3���¶Ե�����Ϊ1������SO3�ļ��Ǹ���
=3�Һ���1���µ��Ӷԣ����ݼ۲���ӶԻ��������жϸ÷��ӿռ乹��ΪV�Σ�SO3�м۵��Ӷ�Ϊ3��û�й¶Ե��ӣ���SO2�м۵�����Ϊ3���¶Ե�����Ϊ1������SO3�ļ��Ǹ���
�ʴ�Ϊ��V�Σ�SO3��SO2��SO3����ԭ�Ӿ�Ϊsp2�ӻ���SO2����һ�Թµ��Ӷԣ����������ų����ø���
��6�����ڸþ�������ԭ����ĿΪ2+4��1/2=4�����ڸþ�����þԭ����ĿΪ��8��1/8+1=2�����Է���ʽΪMgH2��![]() ���ʴ�Ϊ��MgH2��
���ʴ�Ϊ��MgH2��![]() ��
��