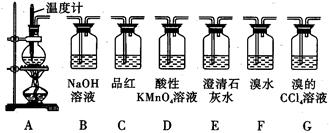
��Ŀ����
��Ԫ���С�����Ԫ�ء�֮�ơ��о���ѧϰС����������ʵ��̽�������е�Ԫ�ش��ڲ��ⶨ���е�Ԫ�صĺ�����
��1������IΪ���գ�������ʱ��____ʢװ����������IIΪ____________________________��
��2��ˮ��ʱͨ��Ҫ������Һ���2��3min��Ŀ����______________________________��
��3������III����ͬѧ�Ƕ���ҺA�е�Ԫ�صĴ�����ʽ���е�̽��ʵ�顣
[�Ʋ�]������IO3����ʽ���ڣ� ����I����ʽ����
[��������]��IO3�����н�ǿ�������ԣ�I2+2S2O32��=2I��+S4O62��
��������Һϡ�����Ƴ�200mL��Һ�����������ʵ��̽������ѡ�Լ���3%H2O2��Һ��KSCN��Һ��FeCl2��Һ��ϡ���ᡣ
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ȡ����ϡ�ͺ����ҺA������ۺ����������ữ����װ���Թ�I��II | ������ | |
| �� | ���Թ�I�м���______ | ������ | ֤��������IO3����ʽ���� |
| �� | ���Թ�II�м���_______ | _______________ | ֤���� ��ʽ���� ��ʽ���� |
��4���������麣���еĵ⺬����
��ȡ20mLϡ�ͺ���ҺA�ֱ�����ƿ���ֱ�����ʽ�ζ��ܵμ�0.01mol/LKMnO4��Һ����Һ����dz��ɫ����I������ΪI2���õ���ҺB��
������ҺB�������ε�����Һ����0.01mol/LNa2S2O3��Һ���ζ����յ㣬�յ�����Ϊ___________����¼���ݣ��ظ��ϲⶨ����١������Σ�����ƽ������Na2S2O3��Һ���ΪVmL�����㺣���е�Ԫ�صİٷֺ���_________________�����������I��II�����еⲻ��ʧ�����ԭ����I��127��
��16�֣���1��������2�֣��� ���ˣ�2�֣�����4�֣�
��2���ӿ캬��������ˮ�е��ܽ⣬��ʹ�ҽ��еĺ������ʾ����ܶ�Ľ�����Һ����2�֣�
��3����FeCl2��Һ�������2��KSCN��Һ������3%H2O2��Һ������Һ������ÿ��2�ֹ�6�֣�
��4����Һ��ɫ�պ���ȥ��30s�ڲ��ָ���ɫ�� ��ÿ��2�֣���4�֣�
��ÿ��2�֣���4�֣�
���������������1���ɺ����ǹ����ȼ�����ʱӦѡ����ʢװ�����ɺ��������˿��Խ���Һ����������Һ�������II�ǹ��ˣ���2���������ʵ��ܽ�����¶����߶����������ҵ�����Һ��е�Ŀ���Ǽӿ캬�����ʵ��ܽ⣬��߹����ĩI�е�Ԫ�صĽ����ʣ���3��ʵ��ڣ�����ҺA�к���IO3����������н�ǿ�������ԣ��ܽ�������������Ϊ�����ӣ���������KSCN��Һ��죬��������IO3�������Թ�I�м���FeCl2��Һ��KSCN��Һ����������ʵ��ۣ�������I����������л�ԭ�ԣ��ܱ�˫��ˮ����Ϊ���ʵ⣬����������������Թ�II�м���3%H2O2��Һ��������Һ������֤������I������4���ζ�ǰ����ҺB�к��е��ʵ⣬������Һ����������ζ�ʱ��I2+2S2O32��=2I��+S4O62�����ζ��յ�ʱ����Һ�����ʵ⣬ֻ�е����ӣ������������Ӳ������������ص�����Ϊ��Һ��ɫǡ����ȥ���Ұ�����ڲ��ָ�Ϊ��ɫ������n=c?V�������εζ�ƽ������V��10��5mol S2O32�����������ӷ���ʽ��ϵ��֮�ȵ������ʵ���֮�ȣ�����ҺB�к���V��10��5/2mol I2�����ݵ�Ԫ���غ�ɵù�ϵʽ��2I����I2���ɴ��ƶ�20mL��ҺA�к���V��10��5mol I������200mL��Һ�к��е�I��ΪV��10��4mol����m(I)="127" V��10��4g�����ݵ�Ԫ�ص������غ㣬���к���127 V��10��4g��Ԫ�أ������е�Ԫ�صİٷֺ���="127" V��10��4/a��100%=1.27V/a%��
���㣺�����ۺ�ʵ�飬�漰��ѧʵ�������������ѧʵ�鷽������ơ��ζ��յ���жϺͻ�ѧ�����֪ʶ��

ʵ�����Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+�����ʣ�Ϊԭ����ȡCaCl2��H2O��CaO2����Ҫ�������£�
��1�������Լ�X��������ҺpHΪ���Ի������Գ�ȥ��Һ��Al3+��Fe3+����������Ҫ�ɷ���___________���Լ�X����ѡ�����е�________�����ţ���
| A��CaO | B��CaCO3 | C��NH3��H2O | D��Ba(OH)2 |
��3����CaCl2��ȡCaO2�ķ�Ӧ�У��¶Ȳ���̫�ߵ�ԭ����_______________��
��4��������װ�òⶨ��ҵ̼��Ƶ���������
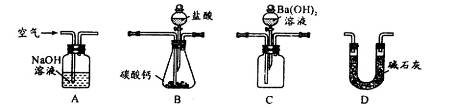
�ټ���װ��B���������õ�ʵ�������__________________________��
�ڰ�A��B��C��D˳�����ӣ�Ȼ���Aװ��ͨ�������Ŀ����_______________��
��װ��D������Ϊ______________________��
��ʵ��ʱ��ȷ��ȡ10.00g��ҵ̼���3�ݣ�����3�βⶨ�����BaCO3������ƽ������Ϊ17.73g������Ʒ��CaCO3����������Ϊ__________________��
��ͼ��ʾ����������ʵ�����Ʊ�������ˮFeCl3����������˳����ȷ����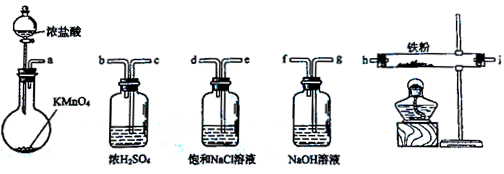
| A��a-b-c-d-e-e-f-g-h |
| B��a-e-d-c-b-h-i-g |
| C��a-d-e-c-b-h-i-g |
| D��a-c-b-d-e-h-i-f |