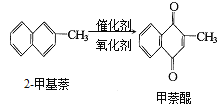
��Ŀ����
����Ŀ����ϩ������ʯ�͵���Ҫ�л�����ԭ�ϣ������ͨ����������һ�����ҵ�ʯ�ͻ�����չˮƽ���������·�ش�

��֪��CH3CHO + O2��CH3COOH
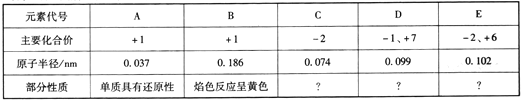
��1���������������������仯����__________������ţ���
�ٷ��� ���ѽ�
��2��A��������__________��
��3����ӦII�Ļ�ѧ����ʽ��________________��
��4��DΪ�߷��ӻ������������������ְ�װ���ϣ���ṹ��ʽ��________________��
��5��E������ζ�����ʣ���ӦIV�Ļ�ѧ����ʽ��______________________��
��6�����й���CH2��CH��COOH��˵����ȷ����__________��
����CH3CH��CHCOOH��Ϊͬϵ��
�ڿ�����NaHCO3��Һ��Ӧ�ų�CO2����
����һ�������¿��Է���ȡ�����ӳɡ�������Ӧ
���𰸡��� �ǻ���OH 2CH3CH2OH +O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O ![]() CH3COOH+CH3CH2OH
CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O �٢ڢ�
CH3COOCH2CH3+H2O �٢ڢ�
��������
��1��ʯ�ͷ���ʱ�����������ʣ��������������仯���ѽ�ʱ���������ʣ��������ڻ�ѧ�仯�����������������������仯���Ǣ٣�
��2����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ����Ҵ��й��������ǻ���
��3����ͭ���������������������£��Ҵ��ܱ���������������ȩ��ˮ����Ӧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��4����ϩ�����Ӿ۷�Ӧ���ɾ���ϩ����ṹ��ʽΪ![]() ��
��
��5����ϩ��ˮ��Ӧ�����Ҵ����Ҵ��ڴ���������������ȩ����ȩ�����������������ᣬ��Ũ���������������������£��Ҵ������ᷢ��������Ӧ��������������ˮ����ӦIV�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��6����CH2=CH-COOH��CH3CH=CHCOOH�ṹ���ƣ����ڷ�����������һ����CH2ԭ���ţ���������ͬϵ���ȷ����CH2=CH-COOH�к����Ȼ����ܺ�̼�����Ʒ�Ӧ���ɶ�����̼����ȷ����CH2=CH-COOH����̼̼˫�����������ں���������ܷ����ӳɷ�Ӧ��ȡ����Ӧ����ȼ�ն�����������Ӧ����ȷ����ѡ�٢ڢۡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
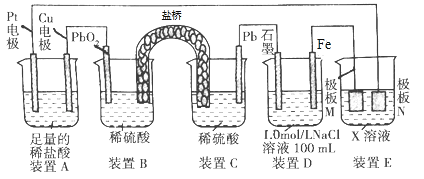
Сѧ��10����Ӧ����ϵ�д�����Ŀ��(1) �Ҵ������������ϵ����Ӽ�����һ�����������ܱ�����Ϊ��ȩ���Ҵ�����Ϊ��ȩ�ķ�Ӧ����ʽΪ________________���Ҵ�_________��������������������ʹ����KMnO4��Һ��ɫ����֪�����£�2.3 g�Ҵ���һ������������Ϻ��ȼ��ǡ����ȫȼ�գ��ų�68.35 kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ_______________________��
(2) ��֪��
��ѧ�� | Si��Cl | H��H | H��Cl | Si��Si |
����/kJ��mol��1 | 360 | 436 | 431 | 176 |
�ҹ辧����ÿ����ԭ�Ӻ�����4����ԭ���γ�4�����ۼ�����ҵ����ȡ�ߴ���ķ�Ӧ����ʽΪ��SiCl4(g)��2H2(g)![]() Si(s)��4HCl(g)���÷�Ӧ�ķ�Ӧ��Ϊ________ kJ��mol��1��
Si(s)��4HCl(g)���÷�Ӧ�ķ�Ӧ��Ϊ________ kJ��mol��1��
(3) ��֪ˮ�ı�����Ϊ4.18��10��3 kJ��g-1��oC-1��10 g�����O2����ȫȼ��������̬SO2���ų���������ʹ500 g H2O���¶���18 ������62.4 ��������Ƶ�ȼ����Ϊ_____________���Ȼ�ѧ����ʽΪ__________________________��