��Ŀ����
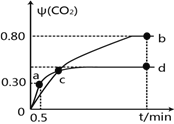
����Ŀ�����з�Ӧ��mA(g)��nB(g) ![]() pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
(1)�÷�Ӧ���淴ӦΪ______(��������������������)��Ӧ����m��n______(����>������������<��)p��
(2)��ѹʹ�����������ʱ��A����������________��(��������������С����������������ͬ)
(3)���ݻ��������B����A��ת����__________��B��ת����________��
(4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮��![]() ��________��
��________��
(5)�����������ƽ��ʱ��������������ʵ���______________________��
���𰸡����� > ���� ���� ��С ��С ����
��������
��֪�ﵽƽ��������¶�ʱ��B��ת���ʱ���������¶ȣ�ƽ�������ƶ�������ӦΪ���ȣ�����Сѹǿ�������ʱ��ƽ�������������ķ����ƶ��������ϵ��C������������С��ƽ�������ƶ�����m+m>p��
��1�����ݷ�����֪������ӦΪ���ȣ����淴ӦΪ���ȷ�Ӧ��m��n>p��
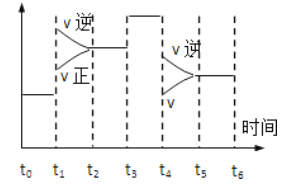
��2����ѹ�ݻ�����ƽ����������ļ�����֮������ķ�Ӧ�����ƶ����������淴Ӧ�����ƶ�����A��������������
��3���ڷ�Ӧ�����м���һ������B����Ӧ��B��Ũ������ƽ��������Ӧ�����ƶ����ٽ�A��ת����A��ת��������B��ת���ʼ�С��
��4������Ӧ���ȣ��������¶�ƽ��������Ӧ�����ƶ���B�����ʵ���Ũ�ȼ�С��C�����ʵ���Ũ�����࣬����ߵ�Ũ�ȱ�ֵ����С��
��5�������Ի�ѧƽ���ƶ�û��Ӱ�죬�����������ƽ�ⲻ�ƶ�����ƽ��ʱ��������������ʵ������䡣

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�����Ŀ��ijʵ��С����0.50 mol��L��1 NaOH��Һ��0.50 mol��L��1 ������Һ�����к��ȵIJⶨ��
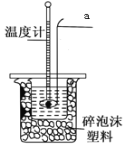
(1)�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ����������a������Ϊ_____________��
(2)д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ(�к���Ϊ57.3 kJ��mol��1)______________��
(3)ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
�� ����д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | __________ |
2 | 27.0 | 27.4 | 26.2 | 31.2 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�� ������Ϊ0.50 mol��L��1 NaOH��Һ��0.50 mol��L��1 ������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4.18kJ��(kg����)��1�����к�����H��________________________��ȡС�����һλ����
�� ����ʵ�����ݽ����57.3 kJ��mol��1��ƫ� ����ƫ���ԭ�����ǣ�����ĸ��________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�