��Ŀ����
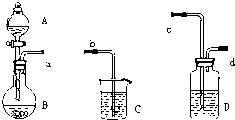
ʵ�����п���������ʾװ����֤���ᡢ̼���ʯ̿�����ߵ��������ǿ����
����˼����ش��������⣺
��1��д��������������ʢװ���Լ���
A______��B______��C______��
��2������Ϊװ��D��װ���Լ�������______��Һ�����������ǣ�______��
��3������ѡ������������װ��һ��װ�ã�����ֱ����֤�����������ʵ�����ǿ���������ӿ�˳��Ϊ��a��______��
��4��ʵ�������ձ���C�п��Թ۲쵽������Ӧ����______��д����Ӧ�Ļ�ѧ����ʽ��______��
����˼����ش��������⣺
��1��д��������������ʢװ���Լ���
A______��B______��C______��
��2������Ϊװ��D��װ���Լ�������______��Һ�����������ǣ�______��
��3������ѡ������������װ��һ��װ�ã�����ֱ����֤�����������ʵ�����ǿ���������ӿ�˳��Ϊ��a��______��
��4��ʵ�������ձ���C�п��Թ۲쵽������Ӧ����______��д����Ӧ�Ļ�ѧ����ʽ��______��

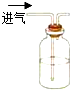
��1����֤���ᡢ̼���ʯ̿�����ߵ��������ǿ����Ӧ����������������̼��Ʒ�Ӧ���ɶ�����̼֤���������Դ���̼�����ԣ�Ȼ�����ɵĶ�����̼ͨ�뱽������Һ�У���֤̼������Դ��ڱ������ԣ�����Aװ��ʢ�ŵ������ᣬBװ��ʢ�ŵ���̼������Һ��Cװ��ʢ�ŵ��DZ�������Һ��
�ʴ�Ϊ�����̼��ƣ���������Һ��
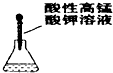
��2������������лӷ��ԣ��ӷ���������Ҳ���Ժͱ�������Һ��Ӧ�����ɱ��ӣ����ż���̼�������뱽�����Ե�ʵ�飬���Խ����ɵĶ�����̼��ͨ������̼��������Һ��ȥ���ᣬ
�ʴ�Ϊ������̼��������Һ����ȥ������̼�е����
��3������ʵ��Ŀ�ģ��ȼ����������Դ���̼�����ԣ�Ȼ������̼�е��������ʳ�ȥ���ٽ����ɵĶ�����̼ͨ�뱽������Һ�У�����̼������Դ��ڱ��ӵ����ԣ�ʵ������������˳��Ϊ��a��c��d��b��
�ʴ�Ϊ��c��d��b��
��4�������Ƶ�������ˮ�е��ܽ�ȼ�С��������̼�ͱ����Ʒ�Ӧ���ɲ�������ˮ�ı��Ӻ�̼�����ƣ����Կ�������������Һ���ֻ��ǣ���Ӧ��ѧ����ʽΪ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
�ʴ�Ϊ����Һ���ֻ��ǣ�C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
�ʴ�Ϊ�����̼��ƣ���������Һ��
��2������������лӷ��ԣ��ӷ���������Ҳ���Ժͱ�������Һ��Ӧ�����ɱ��ӣ����ż���̼�������뱽�����Ե�ʵ�飬���Խ����ɵĶ�����̼��ͨ������̼��������Һ��ȥ���ᣬ
�ʴ�Ϊ������̼��������Һ����ȥ������̼�е����
��3������ʵ��Ŀ�ģ��ȼ����������Դ���̼�����ԣ�Ȼ������̼�е��������ʳ�ȥ���ٽ����ɵĶ�����̼ͨ�뱽������Һ�У�����̼������Դ��ڱ��ӵ����ԣ�ʵ������������˳��Ϊ��a��c��d��b��
�ʴ�Ϊ��c��d��b��
��4�������Ƶ�������ˮ�е��ܽ�ȼ�С��������̼�ͱ����Ʒ�Ӧ���ɲ�������ˮ�ı��Ӻ�̼�����ƣ����Կ�������������Һ���ֻ��ǣ���Ӧ��ѧ����ʽΪ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
�ʴ�Ϊ����Һ���ֻ��ǣ�C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
�����Ŀ
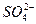 ��Ag+�е�һ�ֻ���
��Ag+�е�һ�ֻ���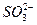

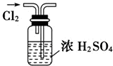 ����Cl2
����Cl2 ������������
������������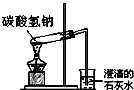 ̼���������ȷֽ�
̼���������ȷֽ� �������ƿ�Ƿ�©ˮ
�������ƿ�Ƿ�©ˮ