题目内容
【题目】草酸是植物(特别是草本植物)常具有的成分,具有广泛的用途。草酸晶体(H2C2O42H2O)无色,熔点为101℃,易溶于水,受热易脱水、升华,170℃以上分解。常温下它的电离常数 K1=5.4×10-2,K2=5.4×10-5。回答下列问题:
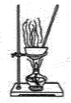
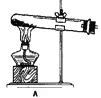
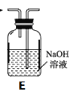
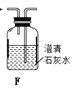
(1)拟用下列装置分解草酸制备少量纯净的CO,其合理的连接顺序为__________(填字母序号)。

![]()

(2)相同温度条件下,分别用3支试管按下列要求完成实验:
试管 | A | B | C |
加入试剂 | 4mL 0.01mol/L KMnO4 1ml 0.1moL/L H2SO4 2mL 0.1mol/L H2C2O4 | 4mL 0.02mol/L KMnO4 1ml 0.1moL/L H2SO4 2mL 0.1mol/L H2C2O4 | 4mL 0.03mol/L KMnO4 1ml 0.1moL/L H2SO4 2mL 0.1mol/L H2C2O4 |
褪色时间 | 28秒 | 30秒 | 不褪色 |
写出试管B中发生反应的离子方程式____________________;上述实验能否说明“相同条件下,反应物浓度越大,反应速率越快” __________(选填“能”或“不能”);简述你的理由:_______________。
(3)设计实验证明草酸为弱酸的方案及其现象均正确的有(___________)(填序号)。
A.室温下,取0.010mol/L的H2C2O4溶液,测其pH=2;
B.室温下,取0.010mol/L的NaHC2O4溶液,测其pH >7;
C.室温下,取pH=a(a<3)的H2C2O4溶液稀释100倍后,测其pH< a+2;
D.标况下,取0.10mol/L的H2C2O4溶液100mL与足量锌粉反应,收集到H2体积为224mL;
(4)为测定某H2C2O4溶液的浓度,取20.00mL H2C2O4溶液于锥形瓶中,滴入2-3滴指示剂,用0.1000mol/L的NaOH溶液进行滴定,并进行3次平行实验,所用NaOH溶液体积分别为19.98mL、20.02mL和22.02mL。
p>①所用指示剂为__________;滴定终点时的现象为_____________________________________;②H2C2O4溶液物质的量浓度为__________;
③下列操作会引起测定结果偏高的是__________(填序号)。
A.滴定管在盛装NaOH溶液前未润洗
B.滴定过程中,锥形瓶震荡的太剧烈,以致部分液体溅出
C.滴定前读数正确,滴定终点时俯视读数
D.滴定前读数正确,滴定终点时仰视读数
【答案】B-E-D 2MnO4- +5H2C2O4 +6H+=2Mn2+ +10CO2↑+8 H2O 能 实验中KMnO4的浓度cB>cA,且其反应速率νB>νA AC 酚酞 锥形瓶内溶液由无色变成(粉)红色,且半分钟内不变化 0.05000mol/L AD
【解析】
(1)由题给信息可知,草酸分解时,草酸为液态,草酸晶体分解生成二氧化碳、一氧化碳、水;
(2)酸性溶液中高锰酸钾溶液氧化草酸生成二氧化碳;硫酸、草酸浓度相同,改变高锰酸钾溶液浓度分析反应速率变化,高锰酸钾溶液浓度越大,反应速率越快;
(3)依据草酸为二元弱酸和草酸氢钠溶液中草酸氢根电离大于水解分析;
(4)①强碱滴定弱酸到反应终点生成草酸钠,生成的为强碱弱酸盐显碱性;
②由H2C2O4—2NaOH建立关系式求解可得;
③滴定操作误差分析可以把失误归结为消耗滴定管中溶液体积的变化分析判断。
(1)由题给信息可知,草酸受热分解时熔化为液态,故选用装置B加热草酸晶体;草酸晶体分解生成二氧化碳、一氧化碳、水,则气体通过装置E吸收二氧化碳,利用排水法收集一氧化碳,其合理的连接顺序为B-E-D,故答案为:B-E-D;
(2)酸性溶液中高锰酸钾溶液氧化草酸生成二氧化碳,反应的离子方程式为:2MnO4-+
5H2C2O4+6H+=2Mn2++10CO2↑+8H2O。保持硫酸、草酸浓度相同,改变高锰酸钾溶液浓度分析反应速率变化,高锰酸钾溶液浓度越大,反应速率越快,实验中KMnO4的浓度cB>cA,且其反应速率νB>νA,上述实验能说明相同条件下,反应物浓度越大,反应速率越快,故答案为:2MnO4-+5H2C2O4+6H+=2Mn2++10CO2↑+8H2O;能;实验中KMnO4的浓度cB>cA,且其反应速率νB>νA;
(3)A、草酸为二元酸,若为强酸电离出氢离子浓度为0.02mol/L,pH小于2,室温下,取0.010mol/L的H2C2O4溶液,测其pH=2,说明存在电离平衡,证明酸为弱酸,故A正确;
B、室温下,0.010mol/L的NaHC2O4溶液中草酸氢根电离大于水解,溶液呈酸性, pH小于7,故B错误;
C、室温下,取pH=a(a<3)的H2C2O4溶液稀释100倍后,测其pH<a+2,说明稀释促进电离,溶液中存在电离平衡,为弱酸,故C正确;
D、标况下,取0.10mol/L的H2C2O4溶液100mL与足量锌粉反应,无论是强酸还是弱酸都收集到H2体积为224mL,故D错误;
故选AC,故答案为:AC;
(4)①强碱滴定弱酸到反应终点生成草酸钠,生成的为强碱弱酸盐显碱性,所以选择酚酞作指示剂,滴入最后一滴锥形瓶内溶液由无色变成(粉)红色,且半分钟内不变化,故答案为:酚酞;锥形瓶内溶液由无色变成(粉)红色,且半分钟内不变化;
②取20.00mLH2C2O4溶液于锥形瓶中,滴入2-3滴指示剂,用0.1000mol/L的NaOH溶液进行滴定,并进行3次平行实验,所用NaOH溶液体积分别为19.98mL、20.02mL和22.02mL,其中22.02mL误差太大,消耗平均体积为20ml,由H2C2O4—2NaOH可得0.020L×c×2=0.1000mol/L×0.020L,解得c=0.05000mol/L,故答案为:0.05000mol/L;
③A、滴定管在盛装NaOH溶液前未润洗,导致溶液浓度减小,消耗标准溶液体积增大,测定结果偏高,故正确;
B、滴定过程中,锥形瓶震荡的太剧烈,以致部分液体溅出,待测液减小,消耗标准溶液体积减小,测定结果偏低,故错误;
C、滴定前读数正确,滴定终点时俯视读数,读取的标准溶液体积减小,测定标准溶液难度偏低,故错误;
D、滴定前读数正确,滴定终点时仰视读数,读取标准溶液体积增大,测定结果偏高,故D正确;
故选AD,故答案为:AD

 仁爱英语同步练习册系列答案
仁爱英语同步练习册系列答案 学习实践园地系列答案
学习实践园地系列答案