��Ŀ����
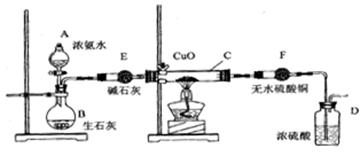
ij����С����ʵ��������ͼ��һЩװ����Ϻ�����ȡ������֤����ijЩ���ʣ�ͬʱ�ռ�����������������ش�
��1��д������ʯ�Һ��Ȼ�立�Ӧ�ư����Ļ�ѧ����ʽ
(2) ��ʵ�����һ��ʱ��۲쵽���ȵ�Ӳ�ʲ����Թ��ں�ɫ����ͭ��ĩתΪ��ɫ��ʢ��ˮ����ͭ�ĸ�����ڳ�����ɫ�����������ij������ܴ��ռ�������������ĵ�����������Щ����д����Ӳ�ʲ����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ�� ,�����Ӧ˵����������____(���ţ�A�����ԣ�B����ԭ�ԣ�C�������ԣ� D�����ȶ���)
(3) ϴ��ƿ��ʢŨ�������Ҫ������__________________
(4) �����ij����ĵ��ܿ��ռ���������ĵ������ռ������ǣ�___________
(���ţ�A�������� B����ˮ�� C���������ռ�)
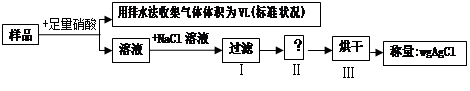
��5��װ��E��������_______________��װ��F��֤��������___________��

��1��д������ʯ�Һ��Ȼ�立�Ӧ�ư����Ļ�ѧ����ʽ
(2) ��ʵ�����һ��ʱ��۲쵽���ȵ�Ӳ�ʲ����Թ��ں�ɫ����ͭ��ĩתΪ��ɫ��ʢ��ˮ����ͭ�ĸ�����ڳ�����ɫ�����������ij������ܴ��ռ�������������ĵ�����������Щ����д����Ӳ�ʲ����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ�� ,�����Ӧ˵����������____(���ţ�A�����ԣ�B����ԭ�ԣ�C�������ԣ� D�����ȶ���)
(3) ϴ��ƿ��ʢŨ�������Ҫ������__________________
(4) �����ij����ĵ��ܿ��ռ���������ĵ������ռ������ǣ�___________
(���ţ�A�������� B����ˮ�� C���������ռ�)
��5��װ��E��������_______________��װ��F��֤��������___________��
��1��2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
��2��2 NH3+3CuO N2+3Cu+3H2O B
N2+3Cu+3H2O B
(3) ����N2 ��ȥ����NH3 ��4��C ��5������NH3 H2O
 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O ��2��2 NH3+3CuO
 N2+3Cu+3H2O B
N2+3Cu+3H2O B(3) ����N2 ��ȥ����NH3 ��4��C ��5������NH3 H2O
�����������2��Ũ��ˮ������ʯ���У���ʯ������ˮ���ȣ�ʹ��ˮ�ֽ�ͬʱ��ʯ����ˮ��Ӧ�����������ƣ�����������Ũ������ʹNH3+H2O
 NH3��H2O
NH3��H2O NH4++OH-����ƽ�������ƶ��������ݳ���������Ũ��ˮ����ʯ�ҿ��Կ����ư�������ʯ�ҵ������Ǹ��ﰱ����E�з���������ԭ����ͭ�ķ�Ӧ����ɫ��ĩ���˵��������ͭ����ˮ����ͭ����˵��������ˮ��ͬʱ���е������ɣ��ɴ˿�д����Ӧ����ʽ2 NH3+3CuO
NH4++OH-����ƽ�������ƶ��������ݳ���������Ũ��ˮ����ʯ�ҿ��Կ����ư�������ʯ�ҵ������Ǹ��ﰱ����E�з���������ԭ����ͭ�ķ�Ӧ����ɫ��ĩ���˵��������ͭ����ˮ����ͭ����˵��������ˮ��ͬʱ���е������ɣ��ɴ˿�д����Ӧ����ʽ2 NH3+3CuO N2+3Cu+3H2O����Ӧ�У�3�۵ĵ�ȫ����Ϊ0�ۣ��������ֻ�ԭ�ԡ�
N2+3Cu+3H2O����Ӧ�У�3�۵ĵ�ȫ����Ϊ0�ۣ��������ֻ�ԭ�ԡ���3��ϴ��ƿ��Ũ����������Ǹ���N2�����ն���İ�������ֹ�����ݳ���Ⱦ������
��4���ռ�����������İ����������ռ���
��5��E�������Ǹ��ﰱ����F��֤��������ˮ������
����������ʵ���ҿ����ư����ķ���������ʯ�һ�����������ƻ��ʯ���еμ�Ũ��ˮ��

��ϰ��ϵ�д�
 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
�����Ŀ