��Ŀ����
Ϊ��СCO2�Ի�����Ӱ��,�ڳ�������̼����ͬʱ,�����ǿ��CO2�������õ��о���T1��ʱ,��9 mol CO2��12 mol H2����3 L�ܱ�������,������ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��
CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��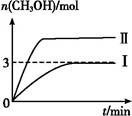
| A�����ߢ��Ӧ�������ı�������ѹǿ |
| B��T2��ʱ,������Ӧƽ�ⳣ��Ϊ0.42,��T2��T1 |
| C����T1��,����ʼʱ�������г���5 mol CO2��5 mol H2��5 mol CH3OH(g)��5 mol H2O(g),���ƽ��ǰv(��)��v(��) |
D����T1��,����ʼʱ����������4.5 mol CO2��6 mol H2,ƽ��ʱ������ѹǿp=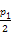 |
D
����

�����й�˵����ȷ����
| A��ʵ������������Ϊ�˼ӿ췴Ӧ���ʣ�����ϡH2SO4���еμ�����Cu(NO3)2��Һ |
| B��Ϊ������¯ˮ���е�CaSO4�������ñ���Na2CO3��Һ���ݣ��ټ��������ܽ� |
C����ӦN2��g��+3H2��g�� 2NH3��g����H<0ƽ��ʱ�������������䣬�����¶ȣ��ٴδﵽƽ��ʱ����ת�������� 2NH3��g����H<0ƽ��ʱ�������������䣬�����¶ȣ��ٴδﵽƽ��ʱ����ת�������� |
| D�����ȷ�Ӧ��TiO2��s��+2Cl2��g��=TiCl4��g��+O2��g������һ�������¿��Է����У���÷�Ӧ�ġ�S<0 |
�����й�˵����ȷ����
A����ˮϡ�ͺ���Һ��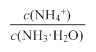 ��ֵ��С ��ֵ��С |
| B��0.1 mol��L��1Na2CO3��Һ������ˮϡ�ͣ�CO32-��ˮ��̶�������Һ��pH��С |
| C����⾫��ͭ�����У����������ļ�������������������һ����� |
| D��298 Kʱ��2H2S(g)��SO2(g)=3S(s)��2H2O(l)���Է����У����䦤H<0 |
��a L NH3ͨ��ij��ѹ���ܱ������У���һ������������ֽ⣬�ﵽƽ��������������b L(�����������ͬ�����²ⶨ)������˵������ȷ����(����)
A��ƽ������ķֽ���Ϊ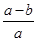 ��100% ��100% |
B��ƽ����������H2���������Ϊ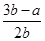 ��100% ��100% |
C����Ӧǰ��������ܶȱ�Ϊ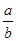 |
D��ƽ��������ƽ��Ħ������Ϊ g g |
��֪��Ӧ�٣�CO(g)��CuO(s)??CO2(g)��Cu(s)�ͷ�Ӧ�ڣ�H2(g)��CuO(s)  Cu(s)��H2O(g)����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2�����¶��·�Ӧ�ۣ�CO(g)��H2O(g)
Cu(s)��H2O(g)����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2�����¶��·�Ӧ�ۣ�CO(g)��H2O(g)  CO2(g)��H2(g)��ƽ�ⳣ��ΪK��������˵����ȷ����(����)
CO2(g)��H2(g)��ƽ�ⳣ��ΪK��������˵����ȷ����(����)
A����Ӧ�ٵ�ƽ�ⳣ��K1��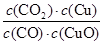 |
B����Ӧ�۵�ƽ�ⳣ��K��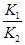 |
| C�����ڷ�Ӧ�ۣ�����ʱ���¶����ߣ�H2Ũ�ȼ�С����÷�Ӧ���ʱ�Ϊ��ֵ |
| D�����ڷ�Ӧ�ۣ����º����£�����ѹǿ��H2Ũ��һ����С |
һ���¶��£���ij2 L�����ܱ������м�������Cu2O��ͨ��0.1 mol H2O(g)��������Ӧ��2H2O(g) 2H2(g)��O2(g)����H����484 kJ��mol��1����ͬʱ�����O2�����ʵ������±���
2H2(g)��O2(g)����H����484 kJ��mol��1����ͬʱ�����O2�����ʵ������±���
| ʱ��/min | 20 | 40 | 60 | 80 |
| n(O2)/mol | 0.001 0 | 0.001 6 | 0.002 0 | 0.002 0 |
����˵����ȷ����(����)
A���ﵽƽ��ʱ����Ҫ��������յ�����Ϊ0.968 kJ
B��ǰ20 min�ڵ�ƽ����Ӧ����v(H2O)��2.5��10��5mol��L��1��min��1
C������c(H2O)���������ˮ�ķֽ���
D��ʹ�ÿ�����С������Cu2O����������ƽ��ʱO2���������
 2C��g��
2C��g�� C(g)+D(g)��5 min���ƽ�⣬��֪�����ʵ�ƽ��Ũ�ȵĹ�ϵΪca(A)��c(B)=c(C)��c(D)�������¶Ȳ��������£����������������Ϊԭ����10����A ��ת����û�з����仯����B��ת����Ϊ( )
C(g)+D(g)��5 min���ƽ�⣬��֪�����ʵ�ƽ��Ũ�ȵĹ�ϵΪca(A)��c(B)=c(C)��c(D)�������¶Ȳ��������£����������������Ϊԭ����10����A ��ת����û�з����仯����B��ת����Ϊ( )