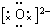��Ŀ����
�ס��ҡ��������ֳ����ĵ��ʣ�X��Y��Z�����ǵĻ��������֮��������ͼ��ʾ��ת����ϵ��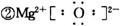
(1)�����Ǿ��л�ԭ�ԵĽ������ʣ�X��Y��Z����һ�������Ӿ��壬���ƶϣ�
��X��Y��Z�к��б�Ԫ�ص���___________����д��Y�ĵ���ʽ___________��
��д��X���Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ��____________________________________________
(2)�����Ǿ��������ԵĻ���ɫ���嵥�ʣ���ͨ���ǰ���ɫ��Һ�壬Y��Z������ͬ�������ӣ�X��Z������ͬ�������ӡ�
��д�������ʵĻ�ѧʽ___________����д��X�������ļ�����Һ����ȫ��Ӧ�����ӷ���ʽ��____________________________________________
(1)��X��Z![]()
![]()
(2)��Fe ��2 Fe2+4Br-+3Cl2====2 Fe3++2Br2+6Cl-
���������⿼��Ԫ�ػ�����֪ʶ��Ҫ��ѧ����һЩ���ͽ���(Mg��Fe)�����ͷǽ���(Cl2��Br2��C)�����ʷdz���Ϥ������������ɫ��Ӧ�������뵽Cl2������������ɫҺ�塱Ӧ�������뵽Br2��������۽���Ӧ�����뵽Fe��

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
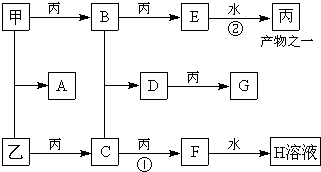
 ��2009?��ģ�⣩��ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��
��2009?��ģ�⣩��ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��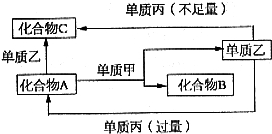 A��B��C����ѧ��ѧ�г���������Ҹ�������Ԫ����ɣ��ס��ҡ��������ֳ����ĵ��ʣ���Щ������͵��ʼ������ͼ��ʾת����ϵ����Щת����ϵ������Ҫʹ�ô��������ش��������⣺
A��B��C����ѧ��ѧ�г���������Ҹ�������Ԫ����ɣ��ס��ҡ��������ֳ����ĵ��ʣ���Щ������͵��ʼ������ͼ��ʾת����ϵ����Щת����ϵ������Ҫʹ�ô��������ش��������⣺