��Ŀ����
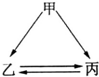
��8�֣���ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��
�������Ƕ����ڽ������ʣ��ҡ����Ƕ����ڷǽ������ʣ�X��Y��Z��ֻ��һ�������Ӿ��壬
���ƶϣ�
��X�ĵ���ʽ��______________________________��
��X���Ӧ�Ļ�ѧ����ʽ____________________________________��
�����������嵥�ʣ���ͨ����Һ�壬Y��Z������ͬ�������ӣ�X��Z������ͬ�������ӣ����ƶϣ�
��д��Z�Ļ�ѧʽ_______________________��
��д��X�������ļ�����Һ����ȫ��Ӧ�����ӷ���ʽ��________________________ _ ��
�Ţ���2�֣� ��2Mg+CO2==2MgO+C��2�֣�
�Ƣ�FeBr3��2�֣� ��3Cl2+2Fe2++4Br-==2Fe3++2Br2+6Cl-��2�֣�
����:�ɿ�ͼ��֪��Ӧ�ף�X������Y�����û���Ӧ��
��1������Ϊ�����ڽ������ҡ����Ƿǽ������ڳ����������û��ǽ����ķ�Ӧ��ֻ��þ�Ͷ�����̼���ϣ��ʼ���þ��X�Ƕ�����̼������̼��Y��MgO��̼�Ͷ�����̼��Ӧ����CO��Z��CO��̼����ȫȼ������CO������������
��2���ڳ����ĵ���Һ����ֻ�е����壨�������������������漰�����������ǵ����塣Y��Z������ͬ�������ӣ�����ת����ϵ��֪X��Ҳ���и�Ԫ�أ��ʸ�Ԫ���DZ�۵Ľ���Ԫ�أ������ı�۽������������ǵ����������Z��FeBr3����X��FeBr2���ܰ�FeBr2��������FeBr3�͵�����ĵ�������Ӧ������������������

 ��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������ǣ�������
��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������ǣ���������C ��H2O2 ��Na ��Fe ��HNO3��
| A�����٢ۢ� | B�����٢ڢ� | C�����٢ڢۢ� | D���٢ڢۢܢ� |
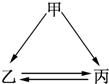 ��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������Ǣ�Fe����HNO3����Na����C��������
��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������Ǣ�Fe����HNO3����Na����C��������| A���٢ڢ� | B���ڢۢ� | C���٢ڢ� | D���٢ڢۢ� |
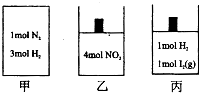 ��ͼ��ʾ�ļס��ҡ����������������зֱ����ķ�ӦΪ��
��ͼ��ʾ�ļס��ҡ����������������зֱ����ķ�ӦΪ���ף�N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol
�ң�2NO2��g��?N2O4
����H2��g��+I2��g��?2HI��g��
�������й�˵������ȷ���ǣ�������
| A���������Ϊ2L������10���Ӧ�ﵽƽ��״̬���ų�����Ϊ55.44U����H2�ķ�Ӧ������0.09mol/��L?s�� | B�����ס����з�Ӧ�ﵽƽ��ʱ�������ͬ�������������������ʵ���������ͬ | C�����ҡ����з�Ӧ�ﵽƽ��ʱ�������ѹǿ����ͬ��������NO2��ת����Ϊ50% | D�����ס��ҡ����з�Ӧ���ﵽƽ��״̬ʱ����������ʵ�ƽ����Է����������䣬�������ʵ���ɫ���䣬���е��¶Ȳ��� |
 ��2009?��ģ�⣩��ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��
��2009?��ģ�⣩��ͼ��ʾ���ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��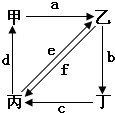 ��ͼ��ʾ���ס��ҡ��������ֱ����CO2��Na2CO3��NaOH��NaHCO3�������ʣ�a��b��c��d��e��f�ֱ��ʾ�������ʼ��ת����ϵ������ͼ���������ʼ��ת����ͨ��һ����Ӧ����ʵ�ֵ��� ��������
��ͼ��ʾ���ס��ҡ��������ֱ����CO2��Na2CO3��NaOH��NaHCO3�������ʣ�a��b��c��d��e��f�ֱ��ʾ�������ʼ��ת����ϵ������ͼ���������ʼ��ת����ͨ��һ����Ӧ����ʵ�ֵ��� ��������