��Ŀ����
16����1��1.5mol H2SO4������147�ˣ����к���3Ħ����ԭ�ӣ�����96����ԭ����2��9.03��1023�������Ӻ�1.5mol�����ӣ�3mol��ԭ�ӣ�15mol���ӣ�9.03��1024������
��3��0.25mol CO2�к���3g̼������£�2.8��CO2�к���0.25mol��ԭ�ӣ�0.5mol CO2�к���3.01��1023��CO2���ӣ�����������22�ˣ�
���� ��1������m=nM�����������������ԭ�����ʵ���Ϊ�����2������ԭ�ӵ����ʵ���Ϊ������ӵ�4�����ٸ���m=nM������Ԫ�ص�������
��2������n=$\frac{N}{{N}_{A}}$���㰱�����ӵ����ʵ������ɻ�ѧʽNH3��֪��Hԭ�ӵ����ʵ����ǰ������ӵ�3����ÿ���������Ӻ���10�����ӡ�10�����ӣ����ӡ��������ʵ���Ϊ������10��������N=nNA���������Ŀ��
��3������n=$\frac{m}{M}$����̼ԭ�����ʵ�����������̼���ʵ�������̼ԭ�����ʵ�����CO2�к���0.25mol��ԭ�ӣ��������̼Ϊ0.125mol������V=nVm���������̼���������n=$\frac{N}{{N}_{A}}$���������̼���ӵ����ʵ������ٸ���m=nM���������̼��������
��� �⣺��1��1.5molH2SO4������Ϊ��1.5mol��98g/mol=147g���ɻ�ѧʽH2SO4��֪��Hԭ�ӵ����ʵ�����������ӵ�2������Hԭ�����ʵ���Ϊ��1.5mol��2=3mol����ԭ�ӵ����ʵ���Ϊ������ӵ�4������Oԭ�����ʵ���Ϊ��1.5mol��4=6mol����Ԫ������Ϊ6mol��16g/mol=96g��
�ʴ�Ϊ��147��3��96��
��2��9.03��1023�������ӵ����ʵ���Ϊ$\frac{9.03��1{0}^{23}}{6.02��1{0}^{23}mo{l}^{-1}}$=1.5mol���ɻ�ѧʽNH3��֪��Hԭ�ӵ����ʵ����ǰ������ӵ�3������Hԭ�ӵ����ʵ���Ϊ1.5mol��3=4.5mol��ÿ���������Ӻ���10�����ӡ�10�����ӣ����������ʵ���Ϊ1.5mol��10=15mol���������ʵ���Ϊ1.5mol��10=15mol��������ĿΪ15mol��6.02��1023mol-1=9.03��1024��
�ʴ�Ϊ��1.5��4.5��15��9.03��1024��
��3��3g̼ԭ�����ʵ���Ϊ$\frac{3g}{12g/mol}$=0.25mol��n��CO2��=n��C��=0.25mol��CO2�к���0.25mol��ԭ�ӣ��������̼Ϊ$\frac{0.25mol}{2}$=0.125mol��������̼���Ϊ0.125mol��22.4L/mol=2.8L��3.01��1023��CO2���ӵ����ʵ���Ϊ$\frac{3.01��1{0}^{23}}{6.02��1{0}^{23}mo{l}^{-1}}$=0.5mol������������0.5mol��44g/mol=22g��
�ʴ�Ϊ��0.35��2.8��0.5��22��
���� ���⿼�����ʵ������йؼ��㣬�Ƚϻ�����ע�����������ʵ���Ϊ���ĵļ��㣮

 ���ݼ���ϵ�д�
���ݼ���ϵ�д����и���ʵ�������ȳ��ֻ��ǵ���D
| ʵ�� | ��Ӧ�¶�/�� | Na2S2O3��Һ | ϡH2SO4 | H2O | ||
| V/mL | c/��mol•L-1�� | V/mL | c/��mol•L-1�� | V/mL | ||
| A | 25 | 5 | 0.1 | 10 | 0.1 | 5 |
| B | 25 | 5 | 0.2 | 5 | 0.2 | 10 |
| C | 35 | 5 | 0.1 | 10 | 0.1 | 5 |
| D | 35 | 5 | 0.2 | 5 | 0.2 | 10 |
| ��A | ��A | ��A | ��A | VA | ��A | V��A | ����0 | |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
��2��DԪ�ص����������������Ʒ�Ӧ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
��3��д��E ��F�γɵ����ֻ�����ĵ���ʽ
 ��
��
��4���õ���ʽ��ʾB��H��Ԫ���γɻ�����Ĺ���
 ��
����5��GԪ�غ�HԪ�����ߺ˵����֮����18��
��6��д��EԪ�ص�ԭ�Ӻ�������Ų�ͼ
 ��HԪ�ص�ԭ�Ӻ�������Ų�ʽ1s22s22p63s22s23p63d104s24p5��
��HԪ�ص�ԭ�Ӻ�������Ų�ʽ1s22s22p63s22s23p63d104s24p5�� | A�� | �����ڷ�Ӧ�еõ����ӣ����ֻ�ԭ�� | |
| B�� | ��1molN2����ʱ����Ӧ��ת�Ƶ�����Ϊ6NA | |
| C�� | ���������뻹ԭ���������֮��Ϊ4��3 | |
| D�� | �÷�Ӧ�и����ʾ����ڷǵ���� |
| A�� | 50mL | B�� | 50.5mL | C�� | 55mL | D�� | 59.5mL |
| A�� | 2��ϩ | B�� | �ױ� | C�� | 1��ϩ | D�� | 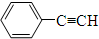 |
| A�� | K+��Na+��Cl-��NO3- | B�� | Mg2+��K+��Cl-��SO42- | ||
| C�� | K+��Na+��Cl-��HCO3- | D�� | K+��Na+��Cl-��SO42- |
 �����ѣ�C4H9OC4H9���Ƕ����ܼ����������л��ϳɷ�Ӧ�ܼ���ijʵ��С��������ͼװ�ã��гֺͼ���װ�þ�ʡ�ԣ��ϳ������ѣ������ķ�ӦΪ��2CH3CH2CH2CH2OH$��_{135��}^{Ũ����}$ C4H9OC4H9+H2O
�����ѣ�C4H9OC4H9���Ƕ����ܼ����������л��ϳɷ�Ӧ�ܼ���ijʵ��С��������ͼװ�ã��гֺͼ���װ�þ�ʡ�ԣ��ϳ������ѣ������ķ�ӦΪ��2CH3CH2CH2CH2OH$��_{135��}^{Ũ����}$ C4H9OC4H9+H2Oʵ�鲽�裺��һ���ݻ���������ƿ�У�����10.9g���൱13.5mL����������2.5mLŨ����ͼ�����ʯ��ҡ�Ⱥ�һ��װ���¶ȼƣ��¶ȼƲ���Һ�����£���һ��װ�Ϸ�ˮ������ˮ�����϶˽�����A�����ڷ�ˮ���ڷ���1.7mLˮ����һ��������������Ȼ��������ƿ����ʯ������С��������У����з�Ӧ����Ӧ�в�����ˮ��A���ռ��ڷ�ˮ�����²㣬�ϲ��л����������ˮ��֧��ʱ�����ɷ���������ƿ����Լ��1.5h��������ƿ�з�ӦҺ�¶ȿɴ�134-136�棬����ˮ��ȫ����ˮ����ʱֹͣ��Ӧ������ӦҺ��ȴ�����º���ʢ��25mLˮ�ķ�Һ©���У��������롢ϴ�Ӻ��ٷ����ᴿ�ɵ�������3.4g����Ӧ��Ͳ������������б����£�
| ҩƷ���� | ��̬ | �ܶȣ�g/mL�� | �۵㣨�棩 | �е㣨�棩 | ˮ���ܽ��� |
| ������ | Һ�� | 0.810 | -89.8 | 118.0 | �� |
| ������ | Һ�� | 0.7689 | -95.3 | 142 | ������ˮ |
| ��ע | �����������ڱ����Ȼ�����Һ�У����������� | ||||
��1������A�������������ܣ�
��2���ϳɴֲ�Ʒʱ��Һ���Լ�����˳�����ȼ������������ټ���Ũ���ᣮ
��3����ӦҺ��ȴ�����º���ʢ��25mLˮ�ķ�Һ©���У���Һ©��ʹ��ǰ��Ҫ��©��ϴ������Һʱ�л����ڷ�Һ©�����ϣ���ϡ����¡����㣮
��4����ʵ������Һ�������л���������1-��ϩ��������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��CH2=CHCH2CH3+Br2��BrCH2CHBrCH2CH3��
��5���л���ֲ���������12mLˮ��8mL5%����������Һ��8mLˮ��8mL�����Ȼ�����Һϴ�ӣ�������������Һϴ�ӵ�Ŀ���dz�ȥ��Ʒ�е����ϴ����ɺ�ͨ�����²��������ᴿ�����ȷ�IJ���˳����cba������ĸ����
a������b������c��������ɫCaCl2
��6����ʵ�����õ��������Ѳ���Ϊ35.34%������С�������λ����
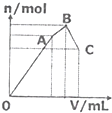 ��100mL 1mol•L-1���������[NH4Al��SO4��2]��Һ����μ���1mol•L-1��Ba��OH��2��Һ��
��100mL 1mol•L-1���������[NH4Al��SO4��2]��Һ����μ���1mol•L-1��Ba��OH��2��Һ��