��Ŀ����
��14�֣�ÿ��2�֣�ij����С������H2��ԭ��ɫ��WO3��ĩ�ⶨW�����ԭ����������ͼ�Dzⶨװ�õ�ʾ��ͼ��A�е��Լ������ᡣ
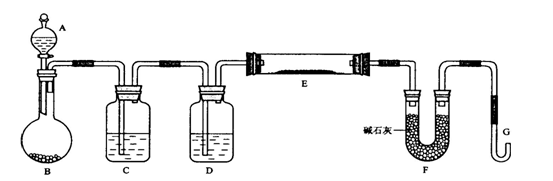
��ش��������⡣
��1��������װ����Լ���C________ �� D________��
��2�����Ӻ�װ�ú�Ӧ����____________________��
��3�������ȷ�Ӧ��E���á�H����ʾ�����͡���Aƿ��εμ�Һ�壨�á�I����ʾ��������������Ӧ���Ƚ��е��ǣ��á�H����I����ʾ��_____________����������֮�仹Ӧ���еIJ�����_____________________��
��4����ʵ���в������������
�ٿ�E�ܵ�����a ��E�ܺ�WO3��������b
�۷�Ӧ��E�ܺ�W�۵�������c����ַ�Ӧ����ȴ�����³�����
�ܷ�ӦǰF�ܼ���ʢ���������d �ݷ�Ӧ��F�ܼ���ʢ���������e
���������ݿ����г�����W�����ԭ�������IJ�����c����ĸ��һ������ʽ�����Բ�д��λ����W�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪����
����ʽ��Ar(W)��____________��
��5����ʵ�����Ҫ��G�������������⣬�ƻ��д�����֮�������������ϴ��������ΪӦ�����θ�����________________________ ��

��ش��������⡣
��1��������װ����Լ���C________ �� D________��
��2�����Ӻ�װ�ú�Ӧ����____________________��
��3�������ȷ�Ӧ��E���á�H����ʾ�����͡���Aƿ��εμ�Һ�壨�á�I����ʾ��������������Ӧ���Ƚ��е��ǣ��á�H����I����ʾ��_____________����������֮�仹Ӧ���еIJ�����_____________________��
��4����ʵ���в������������
�ٿ�E�ܵ�����a ��E�ܺ�WO3��������b
�۷�Ӧ��E�ܺ�W�۵�������c����ַ�Ӧ����ȴ�����³�����
�ܷ�ӦǰF�ܼ���ʢ���������d �ݷ�Ӧ��F�ܼ���ʢ���������e
���������ݿ����г�����W�����ԭ�������IJ�����c����ĸ��һ������ʽ�����Բ�д��λ����W�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪����
����ʽ��Ar(W)��____________��
��5����ʵ�����Ҫ��G�������������⣬�ƻ��д�����֮�������������ϴ��������ΪӦ�����θ�����________________________ ��
��1��ˮ H2SO4(Ũ)
��2�����װ�õ�������
��3������H2�Ĵ���
��4��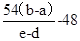 ����д
����д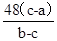 ��
��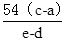 �IJ����֣�
�IJ����֣�
��5����G�ܳ��ڴ���ȼ��ȥβ��
��2�����װ�õ�������
��3������H2�Ĵ���
��4��
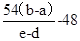 ����д
����д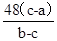 ��
��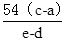 �IJ����֣�
�IJ����֣���5����G�ܳ��ڴ���ȼ��ȥβ��
��1��B��C��Dװ������ȡ�����������������B��Ӧ��п����C��Ӧ�dz�HCl��H2O��D��Ӧ�dz�ȥH2O��Ũ���
��2�������Ӻ�װ�ú�Ӧ���ȼ���װ�õ������ԣ�����Ҫע����������һ���ȷ�����壬�ڶ����Է������ı�ѹǿ��������˵����Ӧ����
��3��װ��E��Ҫ���ȣ����������������ϼ����ױ�ը����Ӧ�ȴ�Aƿ��εμ�Һ����ȡ����������ȡ��������װ���еĿ����ž�������H2�Ĵ��Ⱥ��ټ��ȣ�
��4����W��ԭ����ΪM����
��WO3 + 3H2 W + 3H2O
W + 3H2O
M+48 54
b��a e��d
�������M=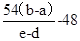
��5����E�з�Ӧ�����ˮ������δ��Ӧ������ͨ�뵽F�У���F�м�ʯ������ˮ����������G���ݳ���������δ��Ӧ�������������������п�ȼ�ԣ�����G�ܳ��ڴ���ȼ��
��2�������Ӻ�װ�ú�Ӧ���ȼ���װ�õ������ԣ�����Ҫע����������һ���ȷ�����壬�ڶ����Է������ı�ѹǿ��������˵����Ӧ����
��3��װ��E��Ҫ���ȣ����������������ϼ����ױ�ը����Ӧ�ȴ�Aƿ��εμ�Һ����ȡ����������ȡ��������װ���еĿ����ž�������H2�Ĵ��Ⱥ��ټ��ȣ�
��4����W��ԭ����ΪM����
��WO3 + 3H2
 W + 3H2O
W + 3H2OM+48 54
b��a e��d
�������M=
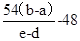
��5����E�з�Ӧ�����ˮ������δ��Ӧ������ͨ�뵽F�У���F�м�ʯ������ˮ����������G���ݳ���������δ��Ӧ�������������������п�ȼ�ԣ�����G�ܳ��ڴ���ȼ��

��ϰ��ϵ�д�
�����Ŀ