��Ŀ����
��ѧ��ӦN2+3H2��2NH3�������仯��ͼ��ʾ��E����ֵ���÷�Ӧ���Ȼ�ѧ����ʽ��( �� )


| A��N2(g)+3H2(g)��2NH3(1)�� ��H��2(a-b-c)kJ��mol��1 |
| B��N2(g)+3H2(g)��2NH3(g)����H��2(b-a)kJ��mol��1 |
C�� N2(g)+ N2(g)+ H2(g)��NH3(1)����H��(b+c-a)kJ��mol��1 H2(g)��NH3(1)����H��(b+c-a)kJ��mol��1 |
D�� N2(g)+ N2(g)+ H2(g)��NH3(g)�� ��H��(a+b)kJ��mol H2(g)��NH3(g)�� ��H��(a+b)kJ��mol |
A
�������������ͼ���֪����Ӧ��������������������������������������Ӧ�Ƿ��ȷ�Ӧ��A��ȷ��C����ȷ������������ǰ�������Ӧ���Ȼ�ѧ����ʽ��N2(g)+3H2(g)��2NH3(g)����H����2(b-a)kJ��mol��1�����B��D���Ǵ���ģ���ѡA��
�����������Ǹ߿��еij���ͼ��������ͼ�������������������Ѷȡ���������������ѧ����ͼ��ʶ�����������Ӧ������������������ѧ��Ӧ�����������ѧ����ѧϰЧ�ʡ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
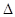 H=��67.7 kJ��mol-1
H=��67.7 kJ��mol-1 F-(aq)+H+(aq)��
F-(aq)+H+(aq)��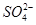 (aq)+
(aq)+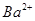 (aq)+2
(aq)+2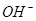 (aq) = BaSO4(s)+2H
(aq) = BaSO4(s)+2H O(l);
O(l);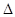 H =
H = 57.3 kJ/mol
57.3 kJ/mol H
H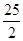 O
O 2HI(g)�� ��H����9.48 kJ/mol��
2HI(g)�� ��H����9.48 kJ/mol��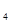 +5O
+5O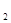 =P
=P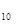 ����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��Pa kJ��mol
����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��Pa kJ��mol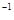 ��P��O b kJ��mol
��P��O b kJ��mol