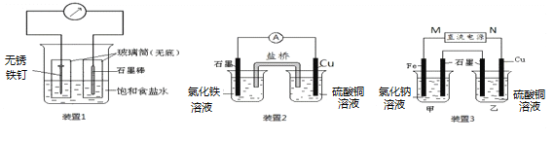
ĚâÄżÄÚČÝ
ˇľĚâÄżˇżĘµŃéĘŇÓĂŇŇ´ĽşÍŨÁňËáÖĆȡŇŇĎ©Ł¬Ć䷴ӦʽΪCH3CH2OH![]() CH2=CH2ˇü+H2OʵŃéʱłŁŇňζȹý¸ß¶řĘąŇŇ´ĽşÍŨÁňËá·´Ó¦ÉúłÉÉŮÁżµÄ¶ţŃő»ŻÁňŁ¬ÓĐČËÉčĽĆĎÂÁĐʵŃéÍĽŇÔČ·ČĎÉĎĘö»ěşĎĆřĚĺÖĐÓĐC2H4şÍSO2Ł®»Ř´đĎÂÁĐÎĘĚ⣺
CH2=CH2ˇü+H2OʵŃéʱłŁŇňζȹý¸ß¶řĘąŇŇ´ĽşÍŨÁňËá·´Ó¦ÉúłÉÉŮÁżµÄ¶ţŃő»ŻÁňŁ¬ÓĐČËÉčĽĆĎÂÁĐʵŃéÍĽŇÔČ·ČĎÉĎĘö»ěşĎĆřĚĺÖĐÓĐC2H4şÍSO2Ł®»Ř´đĎÂÁĐÎĘĚ⣺

Iˇ˘IIˇ˘IIIˇ˘IV×°ÖĂżÉŇÔʢ·ĹµÄĘÔĽÁÓĐŁş
Aˇ˘Ć·şěČÜŇş Bˇ˘NaOHČÜŇş
Cˇ˘Ĺ¨ÁňËá Dˇ˘ËáĐÔKMnO4ČÜŇşŁ¨ĘÔĽÁżÉŇÔÖظ´ĘąÓĂŁ©
Ł¨1Ł©Iˇ˘IIˇ˘IIIˇ˘IV×°ÖĂÖĐËůʢµÄĘÔĽÁ·Ö±đÎŞŁş_________ˇ˘_________ˇ˘_________ˇ˘_________Ł¨ĚîĐňşĹŁ©
Ł¨2Ł©ĘąÓĂ×°ÖĂIIµÄÄżµÄĘÇ_________________ˇŁ
Ł¨3Ł©Č·¶¨ş¬ÓĐŇŇĎ©µÄĎÖĎóĘÇ__________________________ˇŁ
ˇľ´đ°¸ˇżŁ¨1Ł©AŁ»BŁ»AŁ»DŁ»
Ł¨2Ł©łýČĄSO2ŇÔĂâ¸ÉČĹŇŇĎ©µÄĽěŃ飻
Ł¨3Ł©×°ÖĂIIIÖеÄĆ·şě˛»ÍĘÉ«Ł¬×°ÖĂIVÖеÄËáĐÔKMnO4ČÜŇşÍĘÉ«
ˇľ˝âÎöˇż
ĘÔĚâ·ÖÎöŁşI×°ÖĂÖĐŁ¬Ę˘·ĹĆ·şěČÜŇşŁ¬ÄżµÄĘÇŃéÖ¤»ěşĎĆřĚĺÖĐSO2µÄ´ćÔÚŁ»II×°ÖĂÖĐŁ¬Ę˘·ĹNaOHČÜŇşŁ¬ÄżµÄĘÇÎüĘŐ»ěşĎĆřĚĺÖеÄSO2ŇÔĂâ¸ÉČĹŇŇĎ©µÄĽěŃ飻 III×°ÖĂÖĐŁ¬Ę˘·ĹĆ·şěČÜŇşŁ¬ÄżµÄĘÇŃéÖ¤SO2ŇŃłýľ»Ł»IV×°ÖĂÖĐʢ·ĹËáĐÔKMnO4ČÜŇşŁ¬ÄżµÄĘÇĽěŃéŇŇĎ©Ł»Ł¨1Ł©Iˇ˘IIˇ˘IIIˇ˘IV×°ÖĂÖĐËůʢµÄĘÔĽÁ·Ö±đÎŞŁşAˇ˘Bˇ˘Aˇ˘DŁ»Ł¨2Ł©ĘąÓĂ×°ÖĂIIµÄÄżµÄĘÇłýČĄSO2ŇÔĂâ¸ÉČĹŇŇĎ©µÄĽěŃ飻Ł¨3Ł©Č·¶¨ş¬ÓĐŇŇĎ©µÄĎÖĎóĘÇ×°ÖĂIIIÖеÄĆ·şě˛»ÍĘÉ«Ł¬×°ÖĂIVÖеÄËáĐÔKMnO4ČÜŇşÍĘÉ«ˇŁ

 Ěظ߼¶˝Ěʦµă˛¦ĎµÁĐ´đ°¸
Ěظ߼¶˝Ěʦµă˛¦ĎµÁд𰸡ľĚâÄżˇżÓĂÓŇÍĽËůĘľ×°ÖĂ˝řĐĐĎÂÁĐʵŃ飺˝«˘ŮÖĐČÜŇşµÎČë˘ÚÖĐŁ¬Ô¤˛âµÄĎÖĎóÓëʵĽĘĎŕ·űµÄĘÇŁ¨ Ł©

ѡĎî | ˘ŮÖĐÎďÖĘ | ˘ÚÖĐÎďÖĘ | Ô¤˛â˘ÚÖеÄĎÖĎó |
AŁ® | ϡŃÎËá | ĚĽËáÄĆÓëÇâŃő»ŻÄƵĻěşĎČÜŇş | Á˘Ľ´˛úÉúĆřĹÝ |
BŁ® | ŨĎőËá | ÓĂÉ°Ö˝´ňÄĄąýµÄÂÁĚő | ˛úÉúşě×ŘÉ«ĆřĚĺ |
CŁ® | ÂČ»ŻÂÁČÜŇş | ŨÇâŃő»ŻÄĆČÜŇş | ˛úÉú´óÁż°×É«łÁµí |
DŁ® | ˛ÝËáČÜŇş | ¸ßĂĚËáĽŘËáĐÔČÜŇş | ČÜŇşÖđ˝ĄÍĘÉ« |