��Ŀ����
����˵����ȷ����
[ ]
A������ˮ���������������Ũ��Ϊ10-13 mol/L����Һ�У�Ca2+��K+��Cl-��HCO3- ���������ܴ�������
B�������£���0.2 mol/LijһԪ��ROH��Һ��0.1 mol/LHCl��Һ�������ϣ���Ϻ���ҺpH7����c��ROH��>c��R+��
C����Ӧ2N2H4��l����N2��g����2H2��g����H����50.6 kJ /mol��ֻ�ڸ������Է�����
D����֪MgCO3��Ksp=6.82��10-6�������к��й���MgCO3����Һ�У�����c��Mg2+��=
c��CO32-�� ����c��Mg2+�� c��CO32-��=6.82��10-6
c��CO32-��=6.82��10-6
B�������£���0.2 mol/LijһԪ��ROH��Һ��0.1 mol/LHCl��Һ�������ϣ���Ϻ���ҺpH7����c��ROH��>c��R+��
C����Ӧ2N2H4��l����N2��g����2H2��g����H����50.6 kJ /mol��ֻ�ڸ������Է�����
D����֪MgCO3��Ksp=6.82��10-6�������к��й���MgCO3����Һ�У�����c��Mg2+��=
c��CO32-�� ����c��Mg2+��
 c��CO32-��=6.82��10-6
c��CO32-��=6.82��10-6 B

��ϰ��ϵ�д�
�����Ŀ

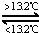 Sn��s���ף���H3=+2.1kJ?mol-1��
Sn��s���ף���H3=+2.1kJ?mol-1��