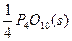ΧβΡΩΡΎ»ί
“―÷ΣΘΚ(1)H2(g)+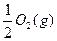 ====H2O(g)
====H2O(g)
ΠΛH1="a" kJΓΛmol-1
(2)2H2(g)+O2(g)====2H2O(g)
ΠΛH2="b" kJΓΛmol-1
(3)H2(g)+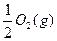 ====H2O(l)
====H2O(l)
ΠΛH3="c" kJΓΛmol-1
(4)2H2(g)+O2(g)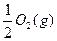 2H2O(l)
2H2O(l)
ΠΛH4="d" kJΓΛmol-1
œ¬Ν–ΙΊœΒ Ϋ÷–’ΐ»ΖΒΡ «Θ® Θ©
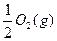 ====H2O(g)
====H2O(g)ΠΛH1="a" kJΓΛmol-1
(2)2H2(g)+O2(g)====2H2O(g)
ΠΛH2="b" kJΓΛmol-1
(3)H2(g)+
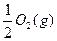 ====H2O(l)
====H2O(l)ΠΛH3="c" kJΓΛmol-1
(4)2H2(g)+O2(g)
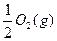 2H2O(l)
2H2O(l)ΠΛH4="d" kJΓΛmol-1
œ¬Ν–ΙΊœΒ Ϋ÷–’ΐ»ΖΒΡ «Θ® Θ©
| AΘ°aΘΦcΘΦ0 | BΘ°bΘΨdΘΨ0 | CΘ°2a=bΘΦ0 | DΘ°2c=dΘΨ0 |
C
H2ΒΡ»Φ…’Ζ¥”ΠΈΣΖ≈»»Ζ¥”ΠΙ BΓΔD¥μΈσΓΘΩΦ¬«H2OΒΡΉ¥Χ§ΦΑΠΛHΘΦ0Θ§Ι aΘΨcΘ§A¥μΈσΓΘΠΛH”κΖΫ≥Χ Ϋ«ΑΟφΒΡΦΤΝΩ ΐ≥…’ΐ±»Θ§Ι 2a=bΘΦ0Θ§C’ΐ»ΖΓΘ

ΝΖœΑ≤αœΒΝ–¥πΑΗ
 Οϊ ΠΒΦΚΫΒΞ‘ΣΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ
Οϊ ΠΒΦΚΫΒΞ‘ΣΤΎΡ©≥ε¥Χ100Ζ÷œΒΝ–¥πΑΗ Οϊ–ΘΟϊΨμΒΞ‘ΣΆ§≤Ϋ―ΒΝΖ≤β ‘ΧβœΒΝ–¥πΑΗ
Οϊ–ΘΟϊΨμΒΞ‘ΣΆ§≤Ϋ―ΒΝΖ≤β ‘ΧβœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
 MnCl2+Cl2Γϋ+2H2O
MnCl2+Cl2Γϋ+2H2O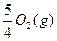 ====
====