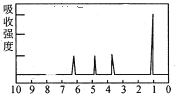
��Ŀ����
����Ŀ���л���A���������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
ʵ�鲽�� | ���ͻ�ʵ����� |
(1)��ȡ9.0g A������ʹ�������������ܶ�����ͬ������������45���� | (1)A����Է�������Ϊ��_____�� |
(2)����9.0g A�������������г��ȼ�գ���ʹ��������λ���ͨ��Ũ���ᡢ��ʯ�ң��������߷ֱ�����5.4g��13.2g�� | (2)A�ķ���ʽΪ��______�� |
(3)��ȡ9.0g A����������̼�����Ʒ�ĩ��Ӧ������2.24L CO2(��״��)���������������Ʒ�Ӧ������2.24L����(��״��)�� | (3)д��A�к��еĹ����ţ�____��_____�� |
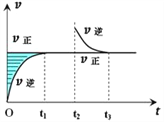
(4)A�ĺ˴Ź���������ͼ��
| (4)A�к���______����ԭ�ӡ� |
(5)��������A�Ľṹ��ʽ__________�� | |
���𰸡�90 C3H6O3 �Ȼ� �ǻ� 4 ![]()
��������
��Է����������ܶȳ����ȣ��ó�A����Է���������ȼ�ղ���ͨ��Ũ��������ˮ��������ʯ������CO2����H2O ��CO2�����������C��H��O�����ʵ��������������ʽ��
(1)�����ܶ�����ͬ������H2��45������֪A����Է�������Ϊ��45��2=90��
(2)���������֪��n��A��=![]() =0.1mol��n��C��=n��CO2��=
=0.1mol��n��C��=n��CO2��=![]() =0.3mol��n��H��=2n��H2O��=2��
=0.3mol��n��H��=2n��H2O��=2��![]() =0.6mol��n��O��=
=0.6mol��n��O��=![]() =0.3mol��N(C):N(H):N(O)= 0.3mol:0.6mol:0.3mol=1:2:1,���л����ʵ��ʽΪCH2O������A����Է�������Ϊ90��֪��A�ķ���ʽΪ��C3H6O3��
=0.3mol��N(C):N(H):N(O)= 0.3mol:0.6mol:0.3mol=1:2:1,���л����ʵ��ʽΪCH2O������A����Է�������Ϊ90��֪��A�ķ���ʽΪ��C3H6O3��
(3)0.1molA��NaHCO3��Ӧ�ų�0.1molCO2����˵A��Ӧ����һ���Ȼ����������������Ʒ�Ӧ������0.1molH2��˵��A�л�����һ���ǻ������Դ�Ϊ��-COOH��-OH��
(4)�˴Ź�����������4�����շ壬���֮��Ϊ1��1��1��3����֪A��Ӧ����4�ֲ�ͬ��������ԭ�ӣ�
(5)����������A�ķ���ʽΪC3H6O3��������к����ǻ����Ȼ����Һ���4��Hԭ�ӣ���A�Ľṹ��ʽΪ��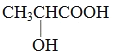 ��
��

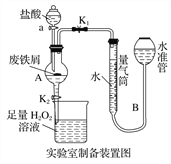
����Ŀ���Ȼ����dz�����ˮ�����������÷���м���Ʊ���ˮ�Ȼ�����ʵ�����Ʊ�װ�ú�ҵ�Ʊ�����ͼ���£�
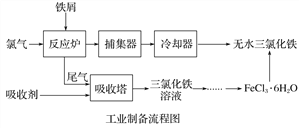
��֪��(1)��ˮFeCl3���۵�Ϊ555 K���е�Ϊ588 K��
(2)����м�е����ʲ������ᷴӦ��
(3)��ͬ�¶�����ˮ���Ȼ�����ˮ�е��ܽ�����£�
�¶�/�� | 0 | 20 | 80 | 100 |
�ܽ��(g/100 g H2O) | 74.4 | 91.8 | 525.8 | 535.7 |
ʵ�����Ʊ������������£�
��.���ɼ�K1���رջ���K2��������a�������μ����
��.������ʱ���رյ��ɼ�K1������K2����A����Һ��ȫ�����ձ���رջ���a��
��.���ձ�����Һ����һϵ�в�����õ�FeCl3��6H2O���塣
��ش�
(1)�ձ���������H2O2��Һ��������_____________________________��
(2)Ϊ�˲ⶨ����м�����������������������С�������������______________��
(3)��FeCl3��Һ�Ƶ�FeCl3��6H2O����IJ��������Ǽ���______________��__________________�����ˡ�ϴ�ӡ����
(4)��д���������з�Ӧ�����ӷ���ʽ��______________________��
(5)�������¶ȳ���673 Kʱ��������Է�������Ϊ325�������Ȼ�������ʵķ���ʽ(���ԭ��������Cl��35.5��Fe��56)Ϊ____________��
(6)FeCl3����������ͨ�����õ������ⶨ����ȡm g��ˮ�Ȼ�����Ʒ������ϡ���ᣬ���Ƴ�100 mL��Һ��ȡ��10.00 mL�������Թ�����KI��Һ����ַ�Ӧ���뼸�ε�����Һ������c mol��L��1 Na2S2O3��Һ�ζ�������V mL(��֪��I2��2S2O32����2I����S4O62��)��
�ٵζ��յ��������________________________________________________��
����Ʒ���Ȼ�������������Ϊ__________________________��