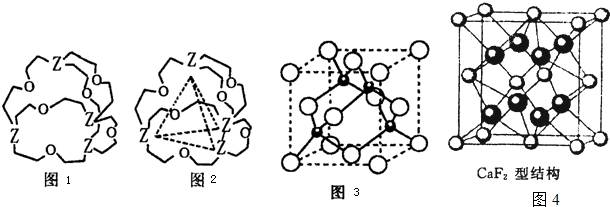
��Ŀ����
�ۻ�ѧ����ѡ�����ʽṹ�����ʣݣ�15�֣�
��Ԫ�����ڱ��У�һϡ������Ԫ��ԭ�ӵ��������ӹ���Ϊ4s24p6������ͬ���ڵ�A��B��C��D����Ԫ�أ����ǵ�ԭ����������������Ϊ2��2��1��7������A��C��Ԫ��ԭ�ӵĴ���������Ϊ8��B��D��Ԫ��ԭ�ӵĴ���������Ϊ18��E��D��Ԫ�ش���ͬ�壬���ڸ���Ԫ���У�E����̬�⻯��ķе���ߡ�
��1��BԪ�������ڱ��е�λ�� ;��2�֣�
DԪ�ػ�̬ԭ�ӵ����Ų�ʽΪ_____________________________����2�֣�
��2��E����̬�⻯����ͬ��Ԫ���зе���ߵ�ԭ���ǣ�
����2�֣�
��3��A��C��Ԫ�ص�һ������ > ������Ԫ�ط��ţ���2�֣�
��4��BԪ�����γɶ�������Ԫ��֮���γ������������ǣ�һ�����ܹ��ṩ�¶Ե��ӵ�ԭ�ӣ���һ���� ��ԭ�ӡ���2�֣�
��5��AԪ�ؿ�����Ԫ���γ����ӻ��������ʽΪ_______________;��2�֣��������ӻ��������ˮ��Ӧ����ѧ����ʽΪ________________________________����3�֣�
��1����4���ڵ�IIB�� ��2�֣�1s22s22p63s23p63d104s24p5��[Ar] 3d104s24p5��2�֣�
��2��E����̬�⻯����Ӽ京�����,�ƻ�����Ҫ�ϸߵ������������۷е�ϸߣ�2�֣�
��3��Ca K��2�֣�
��4�������ܹ����ܹ¶Ե��ӵĿչ����2�֣�
��5�� [H:]-Ca2+[:H] - ��2�֣� CaH2+2H2O=Ca��OH��2+2H2����3�֣�
����: