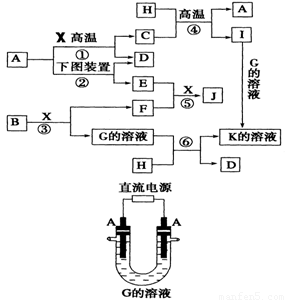
��Ŀ����
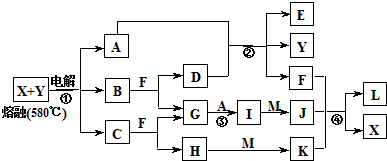
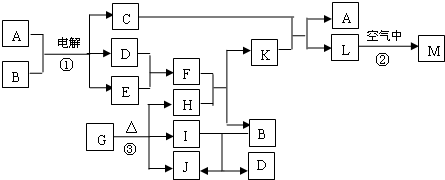
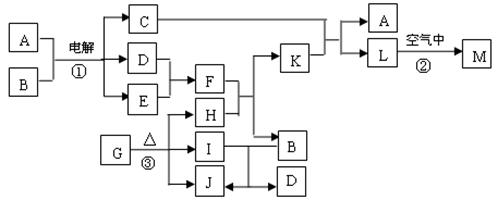
��ͼ��ʾ������֮���ת����ϵ������A��B��C��GΪ���ʡ���Ϊ��ҵ�����г�����Ӧ��E��һ�־���Ư�����õ��Σ�Y�׳��⣬M��һ�����Ի����L��һ�ְ�ɫ������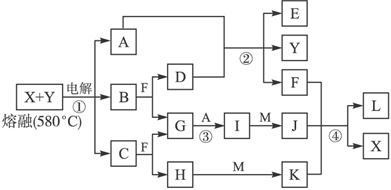
����������й����⣺
(1)M�Ļ�ѧʽ___________��Y��ʵ�����е���;___________________________________(�о�һ��)��
(2)X���۵�Ϊ801 �棬ʵ�ʹ�ҵұ���г�������һ������Y���ۣ����빤ҵ����������Ŀ�ģ�___________________________________����ҵ�����У����������õ��X��F�Ļ������ȡ___________________________________________________��
(3)��Ӧ�ڵĻ�ѧ����ʽ��____________________________________________________��
(4)��Ӧ�ܵ����ӷ�Ӧ����ʽ��________________________________________________��
�����������Կ�ͼ����ʽ��������������������ʡ��ɢ�Ϊ��ҵ�����г�����Ӧ��E��һ�־���Ư�������õ��Σ�Y�׳��⣬�������뵽Ư�۵���ȡ��A�ǵ��ʣ���AΪCl2��DΪCa(OH)2��BΪCa��FΪˮ����M��һ�����Ի�����ܺ�H��I��Ӧ�������Ƴ�H��IΪǿ���ǿ�G��A����I����֪IΪ���ᣬ�²�H����Ϊ�������ƣ��ɴ˵õ�CΪNa��X��Y���Ȼ��ƺ��Ȼ��ƵĻ�����L��һ�ְ�ɫ��������M����������
�𰸣���1��Al2O3 ���������
��2������X��Y�ۻ�ʱ���¶ȣ���Լ��Դ Cl2��H2��NaOH
��3��2Cl2+2Ca(OH)2====CaCl2+Ca(ClO)2+2H2O
��4��Al3++3![]() +6H2O====4Al(OH)3��
+6H2O====4Al(OH)3��

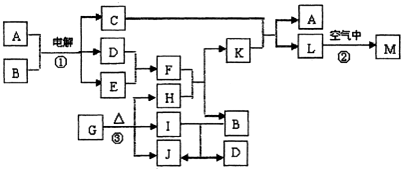
 ��
��