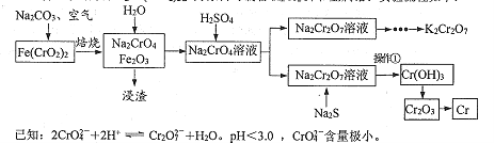
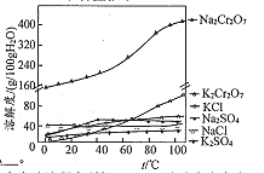
��Ŀ����
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]����������Ͳ�Ѫ�������������������������£�

��֪��TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42-��TiOSO4ˮ���TiO2xH2O����Ϊ���淴Ӧ������ṹ��ʽΪCH3CH(OH)COOH��
��ش�
��1��������з�������������Һ�������IJ�����________________________��
��2��������м��Ŀ��һ�ǻ�ԭ����Fe2(SO4)3������ʹ����TiOSO4ת��ΪTiO2xH2O��������ƽ���ƶ���ԭ�����͵õ�������ԭ��___________________________��
��3�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ____________________________��
��4�������ӷ���ʽ���Ͳ�����м������ܵõ�����������ԭ��_________________��
��5������ܵ����ӷ���ʽ��_________________________________________��
��6������ޱ������һ������նȣ�ԭ��������������ˮ�Լ�___________________��
��7��Ϊ�ⶨ����������þ�����FeSO4��7H2O������������ȡ������Ʒa g������ϡ�������100.00 mL��Һ��ȡ��20.00 mL��Һ����KMnO4��Һ�ζ���������KMnO4����Ӧ����������0.1000 molL-1 KMnO4��Һ20.00 mL�����þ�����FeSO4��7H2O����������Ϊ______����a��ʾ����
���𰸡� ���� TiOSO4+(x+1)H2O![]() TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O
TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+��м��H2SO4��Ӧ��C(H+)������ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1: 4 FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� Fe2++2HCO3-==FeCO3��+H2O+CO2�� ��ֹFe2+������ 13.9/a
TiO2 xH2O��+2H+��м��H2SO4��Ӧ��C(H+)������ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O���� 1: 4 FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� Fe2++2HCO3-==FeCO3��+H2O+CO2�� ��ֹFe2+������ 13.9/a
��������(1) ʵ�ֹ����Һ��ķ����ù��˵ķ������������з�������������Һ�������IJ����ǹ�������ȷ����������
��2�����ݷ�ӦTiOSO4+(x+1)H2O![]() TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O
TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+��֪����м��H2SO4��Ӧ��C(H+)���ͣ�ʹƽ�������ƶ���������TiOSO4ת��ΪTiO2xH2O��������ȷ�𰸣�TiOSO4+(x+1)H2O
TiO2 xH2O��+2H+��֪����м��H2SO4��Ӧ��C(H+)���ͣ�ʹƽ�������ƶ���������TiOSO4ת��ΪTiO2xH2O��������ȷ�𰸣�TiOSO4+(x+1)H2O![]() TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O
TiO2 xH2O��+H2SO4��TiO2++(x+1)H2O![]() TiO2 xH2O��+2H+��м��H2SO4��Ӧ��C(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������
TiO2 xH2O��+2H+��м��H2SO4��Ӧ��C(H+)���ͣ�ʹƽ�������ƶ���TiOSO4ת��ΪTiO2xH2O������
��3�����������ڿ��������������������������ķ���ʽΪ: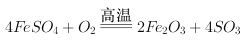 ������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1:4����ȷ�𰸣�1: 4 ��
������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1:4����ȷ�𰸣�1: 4 ��
��4��̼�������ij��������ܽ�ƽ��:![]()
![]() ����������������
����������������![]() �����ᷴӦŨ�Ƚ�����ƽ�������ƶ������ӷ���ʽΪ��FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� ����ȷ���� FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2����
�����ᷴӦŨ�Ƚ�����ƽ�������ƶ������ӷ���ʽΪ��FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2�� ����ȷ���� FeCO3+2CH3CH(OH)COOH== Fe2++2CH3CH(OH)COO-+H2O+CO2����
��5��������ͼ����֪��������������̼����立�Ӧ��̼������,����������Ϊ������̼����ҺBΪ�������Һ����Ӧ���ӷ���ʽΪ: Fe2++2HCO3-==FeCO3��+H2O+CO2������ȷ��Fe2++2HCO3-==FeCO3��+H2O+CO2����
��6��������������ֹFe2+����������ȷ�𰸣� ��ֹFe2+��������
��7���������ӻᱻ���������Һ����Ϊ��������������������ԭΪ+2�۵�������,�辧����FeSO4��7H2
5FeSO47H2O------KMnO4
5��278g 1mol
a��x 0.1��0.02��100/20
��֮x=13.9/a,��ȷ����13.9/a��

����Ŀ�������±���ʾ���ǹ����л���A��B����Ϣ�����ݱ�����Ϣ�ش��������⣺
A | B |
����ʹ������Ȼ�̼��Һ��ɫ �ڷ��ӱ���ģ��Ϊ ������ˮ��һ�������·�Ӧ | ����C��H����Ԫ����� �ڷ������ģ��Ϊ
|
(1)A��������Ȼ�̼��Һ��Ӧ�IJ��������Ϊ______________��
(2)B���������________(�����)��
����ɫ��ζҺ�� ���ж� �۲�����ˮ ���ܶȱ�ˮ�� ����ʹ����KMnO4��Һ����ˮ��ɫ ���κ������¾�����������Ӧ ������ˮ��Ϻ�Һ��ֲ����ϲ�ʳȺ�ɫ
����Ϊ�˲ⶨij�л���C�Ľṹ��������ʵ�飺
�ٽ�2.3 g���л�����ȫȼ�գ�����0.1 mol CO2��2.7 gˮ��
���������Dzⶨ����Է����������õ���ͼ1��ʾ������ͼ��
���ú˴Ź����Ǵ����û�����õ���ͼ2��ʾͼ�ף�ͼ������������֮����1��2��3���Իش��������⣺

(1)�л���C����Է���������________��
(2)�л���C��ʵ��ʽ��________��
(3)д���л���C�Ľṹ��ʽ��_____________________��