��Ŀ����
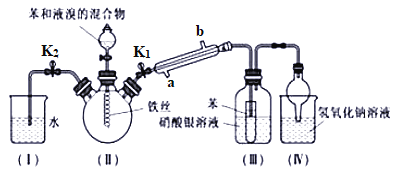
����Ŀ����������(NaNO2)����Ϊ��ҵ�Σ���Ư�ס���Ƶȷ���Ӧ�ù㷺����ľ̿��Ũ���ᡢˮ��ͭΪԭ���Ʊ��������Ƶ�װ����ͼ��ʾ(A�м���װ��ʡ��)��

��֪�������£���2NO+Na2O2=2NaNO2��
��3NaNO2+3HCl=3NaCl��HNO3+2NO��+H2O��
�����������£�NO��NO2-������MnO4-��Ӧ����NO3-��Mn2+��
�밴Ҫ��ش��������⣺
(1)Aװ���з�Ӧ�Ļ�ѧ����ʽΪ______________________________��
(2)Bװ������Ҫ��������______________________________��
(3)����Cװ�ò��������������Ƶķ�����_____________________________��
(4)Dװ���з�Ӧ�����ӷ���ʽΪ_________________________________��
(5)Ԥ��C�з�Ӧ��ʼ�Σ���������NaNO2�⣬�����еĸ�������Na2CO3��________��Ϊ���������Щ�����Ӧ��B��Cװ�ü�����װ��E����E��ʢ�ŵ��Լ�����Ϊ________��
(6)���װ�������Բ�װ��ҩƷ������ʵ�����������ȷ��˳��Ϊ____________(�����)��
a.���ɼУ���װ����ͨ��N2
b.��ȼ�ƾ���
c.��������ƿ�еμ�Ũ����
d.Ϩ��ƾ���
e.�رշ�Һ©��ŷ����
f.ֹͣͨ��N2
(7)���øĽ����װ�ã���7.8gNa2O2��ȫת��Ϊ�������ƣ���������Ҫľ̿______g��
���𰸡�C+4HNO3(Ũ) ![]() CO2��+4NO2��+2H2O ����ɫ������ʧ��ͭƬ�ܽ⣬��Һ���������ܿ�����ɫ����ð�� ȡC�й����������Թ��У��������ᣬ������ɫ���壬��������Ϊ����ɫ 3MnO4-+5NO+4H+=3Mn2++5NO3-+2H2O NaOH ��ʯ�� a��c��e��b��d��f 1.8g
CO2��+4NO2��+2H2O ����ɫ������ʧ��ͭƬ�ܽ⣬��Һ���������ܿ�����ɫ����ð�� ȡC�й����������Թ��У��������ᣬ������ɫ���壬��������Ϊ����ɫ 3MnO4-+5NO+4H+=3Mn2++5NO3-+2H2O NaOH ��ʯ�� a��c��e��b��d��f 1.8g
��������
(1)Aװ��ΪC��ŨHNO3�ڼ���ʱ��Ӧ���ɶ�����̼�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��C+4HNO3(Ũ) ![]() CO2��+4NO2��+2H2O��
CO2��+4NO2��+2H2O��
(2)A��Ӧ�������������Bװ�ã�NO2��B����ˮ��Ӧ�����ᣬ�������ǿ�����ԣ���Cu��Ӧ��������ͭ��NO��ˮ�����Իῴ��B��ʵ������Ϊ������ɫ������ʧ��ͭƬ�ܽ⣬��Һ���������ܿ�����ɫ����ð����
(3)B��Ӧ������NO��Cװ������Na2O2��Ӧ�Ʊ�NaNO2��������֪����3NaNO2+3HCl=3NaCl��HNO3+2NO��+H2O�����Լ���C����NaNO2�ķ����ǣ�ȡC�й����������Թ��У��������ᣬ������ɫ���壬��������Ϊ����ɫ��
(4)��װ��D��ʢ������KMnO4��Һ�������ʾ���ǿ�����ԣ���Ѵ�C���ݳ���NO��������������������ԭ��ΪMn2+������D����Һ��ɫ��dz����Ӧ�����ӷ���ʽΪ��3MnO4-+5NO+4H+=3Mn2++5NO3-+2H2O��
(5)C��Ũ���ᷴӦ�����������г�����NO2�⣬����CO2���壬NO2��B�з�Ӧ��ΪNO����˴�B���ݳ���������NO��CO2��H2O������NO��CO2��H2O������������Ʒ�Ӧ�����������ơ�̼���ƺ��������ƣ�����Ԥ��C�з�Ӧ��ʼ�Σ���������NaNO2�⣬�����еĸ�������Na2CO3������������Ϊ���������Щ�����Ӧ��B��Cװ�ü�����װ��E����E��ʢ�ŵ��Լ���ʯ�ң���������CO2��H2O������
(6)���װ�������Բ�װ��ҩƷ���ȴ��ɼУ���װ����ͨ��N2���ų�װ���еĿ���������������ƿ�еμ�Ũ�������رշ�Һ©��ŷ������Ȼ���ȼ�ƾ��ƣ�����Ӧ������Ϩ��ƾ��ƣ�����ͨ��N2��ʹװ���е�����ȫ������Dװ�ã���ֹ��Ⱦ��������D����Һ����ɫʱ˵��װ������NO����ֹͣͨ��N2���ʲ���˳��Ϊa��c��e��b��d��f��
(7)���ݷ���ʽ��C+4HNO3(Ũ) ![]() CO2��+4NO2��+2H2O��3NO2��H2O=2HNO3+NO��2NO+Na2O2=2NaNO2�ɵù�ϵʽ��3C��12NO2��4NO��2Na2O2��7.8gNa2O2�����ʵ���Ϊ0.1mol�����øĽ����װ�ã���������ȫת��Ϊ�������������ݹ�ϵʽ��֪��ҪC�����ʵ���Ϊ0.15mol������ľ̿������Ϊm(C)=0.15mol��12g/mol=1.8g��
CO2��+4NO2��+2H2O��3NO2��H2O=2HNO3+NO��2NO+Na2O2=2NaNO2�ɵù�ϵʽ��3C��12NO2��4NO��2Na2O2��7.8gNa2O2�����ʵ���Ϊ0.1mol�����øĽ����װ�ã���������ȫת��Ϊ�������������ݹ�ϵʽ��֪��ҪC�����ʵ���Ϊ0.15mol������ľ̿������Ϊm(C)=0.15mol��12g/mol=1.8g��