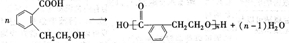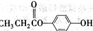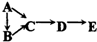��Ŀ����
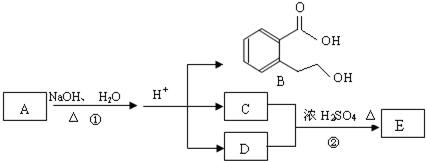
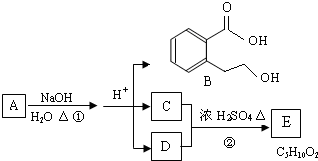
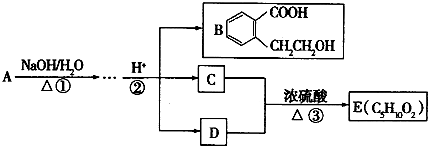
 A��B��C��D��E��Ϊ��ѧ��ѧ�ij������ʻ������֮��ķ�Ӧ��ϵ��ͼ��ʾ��
A��B��C��D��E��Ϊ��ѧ��ѧ�ij������ʻ������֮��ķ�Ӧ��ϵ��ͼ��ʾ��
��1����A�Ƕ�������ԭ�Ӱ뾶����Ԫ�ع��ɵĵ��ʣ�E�ȿ����������ֿ�����NaOH��Һ��E����NaOH��Һ�����ӷ���ʽ��______����ҵ��ұ��A�Ļ�ѧ��Ӧ����ʽ��______��
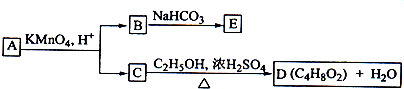
��2����C�ǼȺ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ��ӣ���ʵ������ȡC�Ļ�ѧ����ʽ��______��1mol C��ȫȼ������Һ̬ˮʱ����1300kJ����C��ȫȼ�յ��Ȼ�ѧ����ʽ��______��A����B����Һ��Ӧʱֻ��������C��̼��Ƴ�����ˮ����B�Ļ�ѧʽ��______��
�⣺��1��A�Ƕ�������ԭ�Ӱ뾶����Ԫ�ع��ɵĵ��ʣ�ӦΪNa��E�ȿ����������ֿ�����NaOH��Һ��ӦΪAl��OH��3��
Al��OH��3��NaOH��Һ��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O����ҵұ��Na�õ�����ڵ�NaCl�ķ�������Ӧ�Ļ�ѧ����ʽΪ2NaCl�����ڣ� 2 Na+Cl2����
2 Na+Cl2����
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��2NaCl�����ڣ� 2 Na+Cl2����
2 Na+Cl2����
��2���Ⱥ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ�����C2H2��H2O2��������ȼ�յ���C2H2����AΪCaC2����ˮ��Ӧ����C2H2��Ca��OH��2����B����Һ��Ӧ����C2H2��ˮ��̼��ƣ�
��BΪCa��HCO3��2��
1molC2H2��ȫȼ������Һ̬ˮʱ����1300kJ����2molC2H2��ȫȼ�շų�2600kJ��������
������ȫȼ�յ��Ȼ�ѧ����ʽΪ2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2600 kJ?mol-1��
�ʴ�Ϊ��CaC2+2H2O��C2H2��+Ca��OH��2��2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2600 kJ?mol-1��Ca��HCO3��2��
��������1��A�Ƕ�������ԭ�Ӱ뾶����Ԫ�ع��ɵĵ��ʣ�ӦΪNa��E�ȿ����������ֿ�����NaOH��Һ��ӦΪAl��OH��3����ҵұ��Na�õ�����ڵ�NaCl�ķ�����
��2���Ⱥ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ�����C2H2��H2O2��������ȼ�յ���C2H2����AΪCaC2����ˮ��Ӧ����C2H2��Ca��OH��2����B����Һ��Ӧ����C2H2��ˮ��̼��ƣ�
��BΪCa��HCO3��2��������ʵĻ�ѧ���ʺ���ĿҪ������⣮
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע��Ⱥ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ�����C2H2��H2O2��������ȼ�յ���C2H2��ע���Ȼ�ѧ����ʽ����д��
Al��OH��3��NaOH��Һ��Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O����ҵұ��Na�õ�����ڵ�NaCl�ķ�������Ӧ�Ļ�ѧ����ʽΪ2NaCl�����ڣ�
 2 Na+Cl2����
2 Na+Cl2�����ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��2NaCl�����ڣ�
 2 Na+Cl2����
2 Na+Cl2������2���Ⱥ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ�����C2H2��H2O2��������ȼ�յ���C2H2����AΪCaC2����ˮ��Ӧ����C2H2��Ca��OH��2����B����Һ��Ӧ����C2H2��ˮ��̼��ƣ�
��BΪCa��HCO3��2��
1molC2H2��ȫȼ������Һ̬ˮʱ����1300kJ����2molC2H2��ȫȼ�շų�2600kJ��������
������ȫȼ�յ��Ȼ�ѧ����ʽΪ2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2600 kJ?mol-1��
�ʴ�Ϊ��CaC2+2H2O��C2H2��+Ca��OH��2��2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2600 kJ?mol-1��Ca��HCO3��2��
��������1��A�Ƕ�������ԭ�Ӱ뾶����Ԫ�ع��ɵĵ��ʣ�ӦΪNa��E�ȿ����������ֿ�����NaOH��Һ��ӦΪAl��OH��3����ҵұ��Na�õ�����ڵ�NaCl�ķ�����
��2���Ⱥ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ�����C2H2��H2O2��������ȼ�յ���C2H2����AΪCaC2����ˮ��Ӧ����C2H2��Ca��OH��2����B����Һ��Ӧ����C2H2��ˮ��̼��ƣ�
��BΪCa��HCO3��2��������ʵĻ�ѧ���ʺ���ĿҪ������⣮
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע��Ⱥ��м��Լ��ֺ��зǼ��Լ�����ԭ�ӷ�����C2H2��H2O2��������ȼ�յ���C2H2��ע���Ȼ�ѧ����ʽ����д��

��ϰ��ϵ�д�
�����Ŀ