��Ŀ����
���ܶ�Ϊ1.84g/cm3��H2SO4����������Ϊ98%��Ũ����������250mL 0.5mol/L��ϡ���ᣮ
��1��Ӧѡ��
��2������ȡ��һ�������Ũ�����ܽ���ˮ������IJ���������
��3��Ӧѡ��
��1��Ӧѡ��
10
10
mL��Ͳ��ȡһ�������Ũ���Ὣ���ܽ���ˮ����ѡ�õ���Ͳ���10mL��20mL��50mL��100mL����������Ͳ��ȡŨ�������ʱ���ӣ��������Ƶ�ϡ��������ʵ���Ũ��ƫ��
ƫ��
���ƫ�ߡ�����ƫ�͡�����Ӱ�족������2������ȡ��һ�������Ũ�����ܽ���ˮ������IJ���������
��Ũ���������ձ��ڱ�ע��ʢˮ���ձ��У����ò��������Ͻ���
��Ũ���������ձ��ڱ�ע��ʢˮ���ձ��У����ò��������Ͻ���
��3��Ӧѡ��
250
250
mL������ƿ����������Һ������ƿʹ��֮ǰӦ����©
��©
�������Ƹ���Һ�Ĺ����У������ձ����Ƶ���Һ���й�ϴ��Һȫ��ת��������ƿ���Ӧ������ƿ��ʹ��Һ��Ͼ��ȣ�Ȼ���ذ�����ˮֱ��ע������ƿ��ֱ��Һ���ڿ̶�������1-2cmʱ��������ͷ�ι�
��ͷ�ι�
�μ�����ˮ����Һ�İ�Һ��ǡ����̶������к������ƿ���ã���ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��ת��ҡ�ȶ�Σ�ʹ��Һ��Ͼ��ȣ���������1��ŨH2SO4�����ʵ���Ũ��c=
���ٸ�����Һϡ��ǰ�����ʵ��������������Ũ��������������Ũ��������ѡ����Ͳ�Ĺ��
�������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
�жϣ�
��2��Ũ����ϡ��Ӧ��Ũ�������ձ�����������װ��ˮ���ձ��У����ò��������裮
��3��һ���ݻ�������ƿֻ��������Ӧ�������Һ��������Һ�����������ƿ��
����ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
����ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������˳��
| 100�Ѧ� |
| M |
�������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
| n |
| v |
��2��Ũ����ϡ��Ӧ��Ũ�������ձ�����������װ��ˮ���ձ��У����ò��������裮
��3��һ���ݻ�������ƿֻ��������Ӧ�������Һ��������Һ�����������ƿ��
����ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
����ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������˳��
����⣺��1��ŨH2SO4�����ʵ���Ũ��c=
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=250mL��0.5mol/L����ã�x��6.8������Ӧ��ȡ��Ũ���������6.8mL����ѡ10mL��Ͳ��
��Ͳ��ȡŨ�������ʱ���ӣ���ȡŨ��������С��6.8mL����Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��10��ƫ�ͣ�
��2��Ũ����ϡ�Ͳ���Ϊ����Ũ�������ձ�����������װ��ˮ���ձ��У����ò��������裮
�ʴ�Ϊ����Ũ�������ձ�����������װ��ˮ���ձ��У����ò��������裮
��3��������Һ250mL����������250mL����ƿ������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
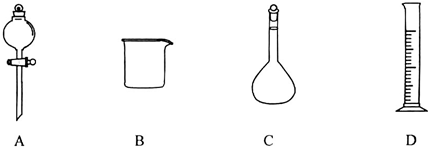
���Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������10mL��Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ�ӣ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
�ʴ�Ϊ��250����©����ͷ�ι�
| 1000��1.84��98% |
| 98 |
��Ͳ��ȡŨ�������ʱ���ӣ���ȡŨ��������С��6.8mL����Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��10��ƫ�ͣ�
��2��Ũ����ϡ�Ͳ���Ϊ����Ũ�������ձ�����������װ��ˮ���ձ��У����ò��������裮
�ʴ�Ϊ����Ũ�������ձ�����������װ��ˮ���ձ��У����ò��������裮
��3��������Һ250mL����������250mL����ƿ������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
���Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������10mL��Ͳ��ȡ���õ���ͷ�ιܣ����������ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ�ӣ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
�ʴ�Ϊ��250����©����ͷ�ι�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
��������ԭ����ע��Ũ�����ϡ�Ͳ�����
| n |
| v |

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
�����Ŀ
 ���ĵġ���������ʻ֤�����ʹ�ù涨����2010��4��1����ʵʩ���¹�涨�Ƽ�һ�ο�12�֣�������ȭ�����Ƽ�����Ϊ�ƺ�ݳ���������ͨ�¹ʵ���Ҫԭ����ͼΪ�����Լ�ʻԱ�Ƿ����ƽ��м�⣮��ԭ�����£�
���ĵġ���������ʻ֤�����ʹ�ù涨����2010��4��1����ʵʩ���¹�涨�Ƽ�һ�ο�12�֣�������ȭ�����Ƽ�����Ϊ�ƺ�ݳ���������ͨ�¹ʵ���Ҫԭ����ͼΪ�����Լ�ʻԱ�Ƿ����ƽ��м�⣮��ԭ�����£�