��Ŀ����
����Ŀ���Ժ�1��̼ԭ�ӵ�����(��CO��CO2��CH4��CH3OH��)Ϊԭ�ϵ�̼һ��ѧ����δ����ѧ��ҵ�ĺ��ģ���Ϊ��ѧ���о�����Ҫ���⡣
(1))��֪CO��H2��CH3OH(g)��ȼ���ȷֱ�Ϊ-283.0 kJ��mol��1��-285.8 kJ��mol��1��-764.5 kJ��mol��1����Ӧ��CO(g)��2H2(g)![]() CH3OH(g)����H��_____����
CH3OH(g)����H��_____����
(2)��T1ʱ�������Ϊ2 L�ĺ��������г������ʵ���֮��Ϊ3 mol��CO��H2��������ӦCO(g)��2H2(g)![]() CH3OH(g)����Ӧ�ﵽƽ��ʱCH3OH(g)���������(��)��n(H2)/n(CO)�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)����Ӧ�ﵽƽ��ʱCH3OH(g)���������(��)��n(H2)/n(CO)�Ĺ�ϵ��ͼ��ʾ��
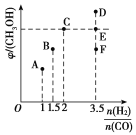
�ٵ���ʼn(H2)/n(CO)��2ʱ������5 min�ﵽƽ�⣬CO��ת����Ϊ0.6����0��5 min��ƽ����Ӧ����v(H2)��______�����˿����������м���CO(g)��CH3OH(g)��0.4 mol���ﵽ��ƽ��ʱH2��ת���ʽ�____(�������С�����䡱)��
�ڵ�n(H2)/n(CO)��3.5ʱ���ﵽƽ���CH3OH���������������ͼ���е�________(����D����E������F��)�㡣
(3)��һ�ݻ��ɱ���ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת����(��)���¶�(T)��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ��
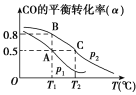
��A��B��C�����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵΪ________��
�����ﵽƽ��״̬Aʱ�����������Ϊ10 L������ƽ��״̬Bʱ���������Ϊ_____L��
(4)�Լ״�Ϊ��Ҫԭ�ϣ��绯ѧ�ϳ�̼��������Ĺ���ԭ����ͼ��ʾ�����Դ�ĸ���Ϊ__(����A������B��)��д�������ĵ缫��Ӧʽ____��
���𰸡���90.1 kJ��mol��1 0.12 mol��L��1��min��1 ���� F KA��KB>KC 2 B 2CH3OH��CO��2e��===(CH3O)2CO��2H��
��������
(1)����CO ��H2��CH3OH��ȼ��������д�ȷ���ʽ�������ø�˹���ɼ���CO(g)��2H2(g)![]() CH3OH(g)������H��
CH3OH(g)������H��
(2) �ٸ���![]() �������ʣ�����Q��K�Ĺ�ϵ�жϷ�Ӧ���ڸ��ݺ��������У�Ͷ�ϱȵ���ϵ���ȣ��ﵽƽ��״̬ʱ����İٷֺ������
�������ʣ�����Q��K�Ĺ�ϵ�жϷ�Ӧ���ڸ��ݺ��������У�Ͷ�ϱȵ���ϵ���ȣ��ﵽƽ��״̬ʱ����İٷֺ������
(3) ����ͬ�¶���ƽ�ⳣ����ȣ�����ͼ��CO��ƽ��ת����(��)���¶����߶���С����֪�����¶�ƽ�������ƶ���
�ڸ���A��B�����ƽ�ⳣ����ȼ�����ƽ��״̬Bʱ�����������
��4���ɽṹʾ��ͼ��֪����������������Ӧ���Ҳ����ԭ��Ӧ����������Ϊ�������Ҳ�Ϊ������
��1����CO��g����H2��g����CH3OH��g����ȼ���ȡ�H�ֱ�Ϊ-283.0 kJ��mol��1��-285.8 kJ��mol��1��-764.5 kJ��mol��1����
��CO��g��+![]() O2��g��=CO2��g����H=-283.0 kJ��mol��1
O2��g��=CO2��g����H=-283.0 kJ��mol��1
��CH3OH��g��+![]() O2��g��=CO2��g��+2 H2O��l����H=-764.5 kJ��mol��1
O2��g��=CO2��g��+2 H2O��l����H=-764.5 kJ��mol��1
��H2��g��+![]() O2��g��=H2O��l����H=-285.8 kJ��mol��1
O2��g��=H2O��l����H=-285.8 kJ��mol��1
�ɸ�˹���ɿ�֪�â�+�ۡ�2-�ڵ÷�ӦCO��g��+2H2��g��=CH3OH��l����
�÷�Ӧ�ķ�Ӧ�ȡ�H=-283.0 kJ��mol��1+��-285.8 kJ��mol��1����2-��-764.5 kJ��mol��1��=��90.1 kJ��mol��1��
(2) �ٵ���ʼn(H2)/n(CO)��2ʱ������ʼn(H2) ��2mol��n(CO)��1mol������5 min�ﵽƽ�⣬CO��ת����Ϊ0.6��
CO(g)��2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ 0.5 1 0
ת�� 0.3 0.6 0.3
ƽ�� 0.2 0.4 0.3
![]() 0.12 mol��L��1��min��1
0.12 mol��L��1��min��1
K![]() 9.375
9.375
���˿����������м���CO(g)��CH3OH(g)��0.4 mol����![]() 7.8125��K�����Է�Ӧ������У��ﵽ��ƽ��ʱH2��ת���ʽ�����
7.8125��K�����Է�Ӧ������У��ﵽ��ƽ��ʱH2��ת���ʽ�����
�ڸ��ݺ��������У�Ͷ�ϱȵ���ϵ���ȣ��ﵽƽ��״̬ʱ����İٷֺ���������Ե�n(H2)/n(CO)��3.5ʱ���ﵽƽ���CH3OH���������������ͼ���е�F�㣻
(3) ����ͬ�¶���ƽ�ⳣ����ȣ�����KA=KB������ͼ��CO��ƽ��ת����(��)���¶����߶���С����֪�����¶�ƽ�������ƶ���ƽ�ⳣ����С������KB��KC����KA��KB>KC��
��A��B�����ƽ�ⳣ����ȣ���B�����������ΪVL
CO(g)��2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ 10 20 0
ת�� 5 10 5
ƽ�� 5 10 5
![]() ��
��
CO(g)��2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ 10 20 0
ת�� 8 16 8
ƽ�� 2 4 8
![]() ��V=2L��
��V=2L��
��4���ɽṹʾ��ͼ��֪����������������Ӧ���Ҳ����ԭ��Ӧ����������Ϊ���������ӵ�Դ���������Ҳ�Ϊ���������ӵ�Դ�ĸ�����BΪ��Դ�ĸ����������Ǽ״���COʧȥ�������ɣ�CH3

����Ŀ���������������������������������ӦΪ��2NO2(g)+O3(g)![]() N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ���ǣ� ��
N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ���ǣ� ��
A | B | C | D |
|
|
|
|
�����¶ȣ� | 0��3s�ڣ���Ӧ����Ϊ�� | t1ʱ����������� | ��ƽ��ʱ�����ı�x����xΪc(O2) |














