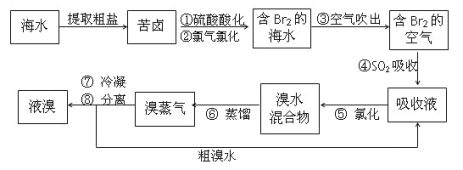
��Ŀ����
����Ŀ����.Ϊ��̽��һ�ֹ��廯����ף�����3��Ԫ�أ�����ɺ����ʣ���Ʋ��������ʵ�飺(��������Ѿ�����ɱ�״���µ������
��ش�
��1��д��������Ļ�ѧʽ________��
��2��д���γ���ҺC�Ļ�ѧ����ʽ��_____________��
��3��д������Aͨ����ҺD�У�������Ӧ�����ӷ�Ӧ����ʽ__________��
��.��������ѧ�����о����������� H2��CH3COOH Ϊԭ�Ϻϳ��Ҵ�����Ӧ��ͬʱ�ᷢ������Ӧ��
��Ӧ��.CH3COOH(g)+2H2(g)![]() CH3CH2OH(g) +H2O(g) ��H1
CH3CH2OH(g) +H2O(g) ��H1
��Ӧ��. CH3COOH(g)+H2(g)![]() CO(g)+CH4(g)+H2O(g) ��H2��0
CO(g)+CH4(g)+H2O(g) ��H2��0
��֪���Ҵ�ѡ������ת���������������Ҵ��İٷֱȡ���ش�
��1����Ӧ��һ�����������Է����У����H1 ___0����������������������
��2��ijʵ���п��� CH3COOH �� H2 ��ʼͶ�ϱ�Ϊ 1��1.5������ͬѹǿ�£�������ͬ��Ӧʱ��������ʵ�����ݣ�
�¶ȣ�K�� | ���� | �����ת���ʣ�%�� | �Ҵ�ѡ���ԣ�%�� |
573 | �� | 40 | 50 |
573 | �� | 30 | 60 |
673 | �� | 55 | 35 |
673 | �� | 40 | 50 |
�����������CH3COOHת��ΪCH3 CH2OHƽ��ת���ʵĴ�ʩ��______��
A ʹ�ô����� B ʹ�ô�����
C ���ͷ�Ӧ�¶� D Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
E ����CH3COOH��H2�ij�ʼͶ�ϱ�
��673K�״��������·�Ӧ���Ѵ�ƽ��״̬����������ת����Ϊ50%���Ҵ���ѡ����40%������ʱ�������Ϊ 1.0L��CH3COOH ��ʼ������Ϊ2.0mol����Ӧ���ƽ�ⳣ�� K= _____��
�۱���ʵ�����ݱ���������ͬ�¶��²�ͬ�Ĵ�����CH3COOHת����CH3CH2OH��ѡ������������Ӱ�죬��ԭ����_________________��
��3����ͼ�зֱ�I�ڴ����ʹ������������������Ӧ����-������ʾ��ͼ��_____

���𰸡�FeAl2S4����Al2FeS4��FeSAl2S3��Al2S3FeS�� Al2O3+2NaOH=2NaAlO2+H2O 2Fe3+ +SO2 +2H2O=2Fe2++SO42��+4H+ �� CD 0.16��5/32 �������ݱ�����ʱ��Ӧδ�ﵽƽ�⣬��ͬ�Ĵ����Է�Ӧ��Ĵ�������ͬ������ڸ�ʱ���¶��Ҵ�ѡ������Ӱ�졣 
��������
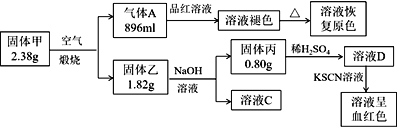
��.������ڿ������������ɵ�����A��ʹƷ����ɫ�����Ⱥ�ָ���ɫ��˵������AΪSO2�������ʵ���Ϊ![]() =0.04mol����������NaOH�ܽ���ˣ��������ϡ�����ܽ�����Һ�еμ�KSCN��Һ���ɫ��˵�������ΪFe2O3�������ʵ���Ϊ
=0.04mol����������NaOH�ܽ���ˣ��������ϡ�����ܽ�����Һ�еμ�KSCN��Һ���ɫ��˵�������ΪFe2O3�������ʵ���Ϊ![]() =0.005mol���������в��ֹ������ܽ���NaOH������Ϊ1.82g-0.8g=1.02g����ΪAl2O3���������ʵ���Ϊ
=0.005mol���������в��ֹ������ܽ���NaOH������Ϊ1.82g-0.8g=1.02g����ΪAl2O3���������ʵ���Ϊ![]() =0.01mol����ʱ������к��е�����Ԫ�ص�������Ϊ0.04mol��32g/mol+0.005mol��2��56g/mol+0.01mol��2��27g/mol=2.38g���������ΪFe2O3��Al2O3�Ļ���
=0.01mol����ʱ������к��е�����Ԫ�ص�������Ϊ0.04mol��32g/mol+0.005mol��2��56g/mol+0.01mol��2��27g/mol=2.38g���������ΪFe2O3��Al2O3�Ļ���
��.(1)��H-T��S��0�ķ�Ӧ���Է����У�
(2)�ٽ��ƽ����ƶ����ط�����һ�㵱ƽ���������ʱ������߷�Ӧ���ת���ʣ�
��673K ʱ���ڼ״��������·�Ӧ���Ѵ�ƽ��״̬����ʱ�������Ϊ 1.0L���� CH3COOH ��ʼ������Ϊ 2.0mol��������м�����ʽ�õ�ƽ��Ũ�ȣ���Ӧ���ƽ�ⳣ�� K=![]() ��
��
�۲�ͬ�Ĵ�����Ч�ʲ�ͬ��
(3)����������ɽ��ͷ�Ӧ�Ļ�ܣ�������Խǿ�����Խ�ͣ�����Ӧ�Ȳ��䡣
��. ������ڿ������������ɵ�����A��ʹƷ����ɫ�����Ⱥ�ָ���ɫ��˵������AΪSO2�������ʵ���Ϊ![]() =0.04mol����������NaOH�ܽ���ˣ��������ϡ�����ܽ�����Һ�еμ�KSCN��Һ���ɫ��˵�������ΪFe2O3�������ʵ���Ϊ
=0.04mol����������NaOH�ܽ���ˣ��������ϡ�����ܽ�����Һ�еμ�KSCN��Һ���ɫ��˵�������ΪFe2O3�������ʵ���Ϊ![]() =0.005mol���������в��ֹ������ܽ���NaOH������Ϊ1.82g-0.8g=1.02g����ΪAl2O3���������ʵ���Ϊ
=0.005mol���������в��ֹ������ܽ���NaOH������Ϊ1.82g-0.8g=1.02g����ΪAl2O3���������ʵ���Ϊ![]() =0.01mol����ʱ������к��е�����Ԫ�ص�������Ϊ0.04mol��32g/mol+0.005mol��2��56g/mol+0.01mol��2��27g/mol=2.38g���������ΪFe2O3��Al2O3�Ļ���
=0.01mol����ʱ������к��е�����Ԫ�ص�������Ϊ0.04mol��32g/mol+0.005mol��2��56g/mol+0.01mol��2��27g/mol=2.38g���������ΪFe2O3��Al2O3�Ļ���
(1)�ɷ���֪����������Ԫ����Fe��Al��S�����ߵ����ʵ���֮��Ϊ0.01mol��0.02mol��0.04mol=1:2:4������Ļ�ѧʽΪFeAl2S4��
(2)����������NaOH��Һ����ƫ�����ƣ�������Ӧ�Ļ�ѧ����ʽΪAl2O3+2NaOH=2NaAlO2+H2O��
(3)����AΪSO2���壬��ҺD����Fe3+��ͨ��SO2����������ԭ��Ӧ��������Һ������Fe2+��SO42-��������Ӧ�����ӷ�Ӧ����ʽ2Fe3+ +SO2 +2H2O=2Fe2++SO42��+4H+��
��.(1)��CH3COOH(g)+2H2(g)�TCH3CH2OH(g)+H2O(g)��H1����Ӧ��S��0��������H-T��S��0������H1��0��
(2)����֪CH3COOH(g)+2H2(g)![]() CH3CH2OH(g) +H2O(g) ��H1��0��
CH3CH2OH(g) +H2O(g) ��H1��0��
A��ʹ�ô����ײ�Ӱ��ƽ����ƶ��������CH3COOHת��ΪCH3 CH2OHƽ��ת���ʣ���A
B��ʹ�ô����Ҳ�Ӱ��ƽ����ƶ��������CH3COOHת��ΪCH3 CH2OHƽ��ת���ʣ���B����
C�����ͷ�Ӧ�¶�ƽ�������ƶ��������CH3COOHת��ΪCH3 CH2OHƽ��ת���ʣ���C��ȷ��
D��Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ�ȣ��൱������ѹǿ��ƽ��������Ӧ�ƶ��������CH3COOHת��ΪCH3 CH2OHƽ��ת���ʣ���D��ȷ��
E������CH3COOH��H2�ij�ʼͶ�ϱȣ����൱������CH3COOH��Ũ�ȣ�ƽ��������Ӧ�����ƶ��������H2��ת���ʣ���CH3COOHת��ΪCH3 CH2OHƽ��ת���ʽ��ͣ���E����
�ʴ�ΪCD��
�ڿ��� CH3COOH �� H2 ��ʼͶ�ϱ�Ϊ 1��1.5��673K ʱ���ڼ״��������·�Ӧ���Ѵ�ƽ��״̬������ת����50%����ʱ�������Ϊ 1.0L���� CH3COOH ��ʼ������Ϊ 2.0mol���Ҵ���ѡ����Ϊ0%����Ӧ���У�
CH3COOH(g)+2H2(g)�TCH3CH2OH(g)+H2O(g)
��ʼ��(mol/L)2 3 0 0
�仯��(mol/L)2��50%=1 2 1��0.4 1��0.4
ƽ����(mol/L) 1 1 0.4 0.4
��Ӧ���ƽ�ⳣ�� K=![]() =0.16��
=0.16��
�۱���ʵ�����ݱ�������ʱ��Ӧδ�ﵽƽ�⣬��ͬ�Ĵ����Է�Ӧ��Ĵ�������ͬ������ڸ�ʱ���¶�CH3COOHת����CH3CH2OH��ѡ������������Ӱ�죻
(3)�ӱ������ݷ������ڴ����ҵ������£��Ҵ���ѡ���Ը���˵�������ҶԷ�Ӧ���Ч�����ã������ܽ��ͷ�Ӧ�Ļ�ܣ�˵��ʹ�ô����ҵķ�Ӧ�����л�ܸ��ͣ���ͼΪ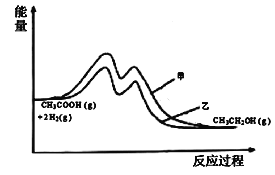 ��
��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�����Ŀ�������ŷŵ�β��Ϊ������Ⱦ��֮һ,Ŀǰ,���������»�ѧԭ����������β��:2NO+2CO![]() 2CO2+N2��
2CO2+N2��
(1)д��CO2�Ľṹʽ____________��
(2)һ��������,���ݻ��̶��������н���������Ӧ,COŨ����ʱ���ϵ��ͼ��ʾ:

��Ӧ����v(a)��v(b)��v(c)�Ĵ�С��ϵ��_______��
(3)Ϊ�о������߸�ת�����̷�Ӧ����,ij���������������ʵ��̽����
�����ϲ��ģ�A.��ͬ�Ĵ�����ͬһ��Ӧ�Ĵ�Ч�ʲ�ͬ;
B.ʹ�õ�������ͬ�Ĵ���ʱ,�����ıȱ�����Դ�Ч����Ӱ�졣
��ʵ����ƣ�������Ϊ̽��ijЩ�������������β��ת����Ӧ���ʵ�Ӱ�����,��������¶Ա�ʵ�飺
ʵ���� | ʵ��Ŀ�� | T/�� | NO��ʼŨ��/(mol/L) | CO��ʼŨ��/(mol/L) | ͬ�ִ����ıȱ����/(m2/g) | c(CO)����ʱ���õ�ʱ��/min |
�� | ����ʵ�� | 280 | 6.50��10-5 | 4.00��10-3 | 80 | t |
�� | 280 | 6.50��10-3 | 4.00��10-3 | 120 | 0.5t | |
�� | 360 | 6.50��10-3 | 4.00��10-3 | 80 | 0.2t |
�����ۣ�
�� ʵ���Ţ��ʵ��Ŀ��Ϊ______________��
�� ������̽���������������β��ת����Ӧ���ʵ�Ӱ�����һ������_______, ��______��Ӧ���ʽ�_______(��������������С��������Ӱ����)��