��Ŀ����
����Ŀ������14.4gCO��CO2�Ļ�����壬�ڱ�״���������Ϊ 8.96L����ش��������⣺
��1���û�������ƽ��Ħ������ Ϊ _____��
��2���û������ ��̼ԭ�ӵĸ��� Ϊ _____������N A��ʾ�����ӵ�������ֵ��
��3�����û����������ͨ������ͼ��ʾװ�ã�����ռ���������������ڱ�״���²ⶨ�� ��
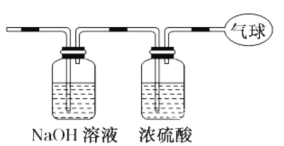
�������ռ����������Ħ������ _____��
�� �������ռ���������ĵ�������Ϊ _____������ NA�� ʾ�����ӵ�������ֵ ��
�� ������������ռ������������ ��Ϊ _____L��
���𰸡�36g/mol0.4NA28g/mol2.8NA4.48L
��������
��1����M=m/n��n=V/Vm���㡣
��2������������CO�����ʵ���Ϊxmol��CO2�����ʵ���Ϊymol�����ݻ��������Ϊ14.4g��������������Ϊ8.96L���з��̼��㣻
��3���ٽ������������ͨ����ͼװ�ã���CO2�ᱻNaOH��Һ���գ�ʣ��CO����Ũ�������������������ռ������Ǹ��﴿����CO���壻
��һ��CO����14�����ӣ��ɣ�1�������CO�����ʵ���������ӵ����ʵ�������Ŀ��
�������е�����ΪCO���������V=nVm�����㣻
��1��������������ʵ���Ϊ8.96L/22.4L��mol��1=0.4mol���û�������ƽ��Ħ������ΪM=14.4g/0.4mol=36g/mol��
��2������������CO�����ʵ���Ϊxmol��CO2�����ʵ���Ϊymol������ݻ���������Ϊ14.4g�ɵã�28x+44y=14.4 �٣��ɵã�x+y=0.4 �ڣ���٢ڵã�x=0.2mol��y=0.2mol������CO��CO2�о���1��̼ԭ�ӣ���0.2molCO��0.2molCO2�й���0.4molCԭ�Ӽ�0.4NA����
��3���������������ͨ����ͼװ�ã���CO2�ᱻNaOH��Һ���գ�ʣ��CO����Ũ�������������������ռ������Ǹ��﴿����CO���壮
���������ռ���������ΪCO����һ�����ʵ�Ħ����������ֵ�ϵ��ڸ����ʵ���Է������������ռ��������Ħ������Ϊ28g��mol��1��
��һ��CO����14�����ӣ��ɣ�2�������CO�����ʵ���Ϊ0.2mol������ӵ����ʵ���Ϊ0.2mol��14=2.8mol����������Ϊ2.8NA����
�������е�����ΪCO�������V=nVm=0.2mol��22.4L��mol��1=4.48L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����˾ƥ�֣�����ˮ������![]() ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ��������
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128����135����ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ��������

�Ʊ����������������£�
![]()
��Ҫ�Լ��Ͳ�Ʒ�������������±���ʾ��
���� | ��Է������� | �۵��е㣨���� | ˮ |
ˮ���� | 138 | 158���۵㣩 | �� |
������ | 102 | 139.4���е㣩 | ��ˮ�� |
����ˮ���� | 180 | 135���۵㣩 | �� |
�����������Ϣ�ش��������⣺
��1���Ʊ���˾ƥ��ʱ��Ҫʹ�ø����������ԭ����________________________________��
��2���ϳɰ�˾ƥ��ʱ��������ļ��ȷ�����______________________��
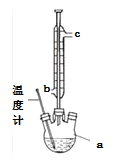
��3���ᴿ�ֲ�Ʒ�������£����Ȼ���װ����ͼ��
�ٷ�ʯ��������__________________________________��
������ˮ������������________________������b������c������
��ʹ���¶ȼƵ�Ŀ����_____________________________________________________��
��4����ʵ����ԭ��������2.0gˮ���ᡢ5.0mL��������![]() �������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ__________________���ðٷ�����ʾ��С�����һλ����
�������ճƵò�Ʒ����Ϊ2.2g������������ˮ����IJ���Ϊ__________________���ðٷ�����ʾ��С�����һλ����
����Ŀ���������Ǻ���Ҫ�Ľ������ѳ�Ϊ������������Ҫ�IJ��ϡ��ش�����������
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ__________________�������Ѿ���������ͼ��ʾ����������Ϊa=b= 295.08pm��c=468.55pm����=��=90%��y= 120%��������Ϊ______________�ѻ�(��ѻ���ʽ)��
��2�������ڵ�þ������л�ԭTiCl4�ɵõ���յĺ����ѡ���֪TiCl4��ͨ�����������ɫҺ�壬�۵�Ϊ-23�棬�е�Ϊ136�棬��֪TiCl4Ϊ____________�� �塣
��3��ͨ��X-����̽��KCl��CaO��TiN������NaCl����ṹ���ƣ���֪�������Ӿ���ľ���������������
���Ӿ��� | KCl | CaO |
������(kJ/mol) | 715 | 3401 |
����KCl������С��CaO��ԭ����_______________��
�ѿ���C��N��O��Ԫ���γɶ�Ԫ�����C��N��OԪ�صĵ縺���ɴ�С��˳����________��
(4)���ѿ���Ľṹ������ͼ��ʾ������Ļ�ѧʽΪ_________________��
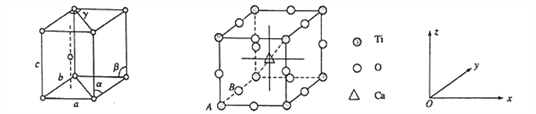
�����е�ԭ�ӿ���x��y��z��ɵ����������������ھ����е�λ�ã���Ϊԭ�����ꡣ��֪ԭ������ΪA(0��0��0)��B(0��1/2��0)����Ca ���ӵ�ԭ������Ϊ______________��
��5��Fe���ġ��á�������ͬ�������壬�侧���ṹ����ͼ��ʾ��

�٦ġ������־��徧������ԭ�ӵ���λ��֮��Ϊ_______________________��
����Feԭ�Ӱ뾶Ϊrpm��NA��ʾ�����ӵ�������ֵ������-Fe���ʵ��ܶ�Ϊ________g/cm3(�г���ʽ����)��
