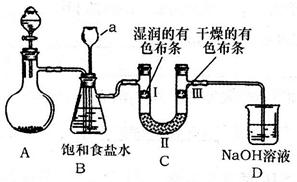
��Ŀ����
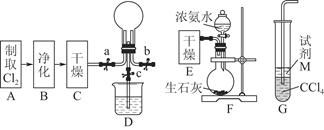
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ���ó�ʪ��KClO3�Ͳ��ᣨH2C2O4����60ʱ��Ӧ�Ƶá�ijѧ����������ͼ��ʾ��װ��ģ����ȡ���ռ�ClO2��

��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��BҲ���������¶ȿ���װ�ã�Ӧ���� (ѡ���ˮԡ������ˮԡ��)װ�á�
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2�IJ������裺
�� ���� ����ϴ�ӣ��ܸ��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ�����ͬ���ӵ����غ�ĽǶȽ�����ԭ���� ��
ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00 mL��ϡ�ͳ�100.00 mL��������ȡV1 mL�������뵽��ƿ�У�
����2������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol��L��1 Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪2 Na2S2O3 + I2 ��Na2S4O6 + 2NaI��
��5���жϵζ��յ������ ��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����

��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��BҲ���������¶ȿ���װ�ã�Ӧ���� (ѡ���ˮԡ������ˮԡ��)װ�á�
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2�IJ������裺
�� ���� ����ϴ�ӣ��ܸ��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ�����ͬ���ӵ����غ�ĽǶȽ�����ԭ���� ��
ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00 mL��ϡ�ͳ�100.00 mL��������ȡV1 mL�������뵽��ƿ�У�
����2������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol��L��1 Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪2 Na2S2O3 + I2 ��Na2S4O6 + 2NaI��
��5���жϵζ��յ������ ��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
��1��2KClO3+ H2C2O4  K2CO3+CO2��+2ClO2��+H2O ��2�֣�
K2CO3+CO2��+2ClO2��+H2O ��2�֣�
��2���¶ȼơ�������ˮԡ��������2�֣�
��3�������ᾧ�������ȹ��ˡ�������2�֣�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ���������������2�֣�
��5���ӵ����һ��ʱ����Һ����ɫͻȻ����ɫ��Ϊ��ɫ���Ұ���Ӳ��仯��2�֣�����135cV2/V1��2�֣�
 K2CO3+CO2��+2ClO2��+H2O ��2�֣�
K2CO3+CO2��+2ClO2��+H2O ��2�֣���2���¶ȼơ�������ˮԡ��������2�֣�
��3�������ᾧ�������ȹ��ˡ�������2�֣�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ���������������2�֣�
��5���ӵ����һ��ʱ����Һ����ɫͻȻ����ɫ��Ϊ��ɫ���Ұ���Ӳ��仯��2�֣�����135cV2/V1��2�֣�
�����������1��A�з�Ӧ������K2CO3��ClO2��CO2��,����60�棬����غͲ��ᷴӦ����̼��ء�������̼���������Ⱥ�ˮ����Ӧ����ʽΪ��2KClO3+H2C2O4=K2CO3+CO2��+2ClO2��+H2O;
��2��Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���60��ʱ��Ӧ�Ƶã�Ӧ��ˮԡ���ȣ������ձ�����ˮԡ�������������ȵ��۵�ϵͣ�Ϊ�ռ��������ȣ�Ӧ�ڽϵ��¶��½��У�����Ӧ�ò��ñ�ˮԡ���ʴ�Ϊ����ˮԡ�� �ձ�����ˮԡ�������¶ȼƣ�
��3��������ϢNaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ������¶������ܽ����������벹���NaClO2��Һ���Ƶ�NaClO2�IJ�������Ϊ�����ᾧ�ͳ��ȹ��ˡ�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ�
��5���жϵζ��յ������Ϊ�ζ��յ�ʱ��I2��ȫ��Ӧ����Һ����ɫ��Ϊ��ɫ���ʴ�Ϊ����ɫ��Ϊ��ɫ�Ұ���Ӳ��仯��
��6����ԭClO2��Һ��Ũ��Ϊx��


��ϰ��ϵ�д�
�����Ŀ