��Ŀ����
�ϳɰ��Ի�ѧ��ҵ������ҵ������Ҫ���壮
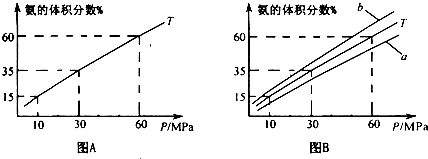
��1����ϳ����а�1��4�����ʵ���֮�ȳ���N2��H2���а��ĺϳɣ�ͼAΪT0Cʱƽ�������а��������������ѹǿ��P���Ĺ�ϵͼ��
��д����ҵ�Ϻϳɰ��Ļ�ѧ����ʽ______��
��ͼA�а������������Ϊ15.00%ʱ��H2��ת����=______��
��ͼB��T=500��C���¶�Ϊ4500C��Ӧ��������______��ѡ����ĸ��a����b������ѡ���������______
����ͼ��֪������ѹǿ�����ԭ�ϵ������ʣ�������ʵ�ʿ�������ѹǿ������������______��д��һ�����ɣ���
��2���ϳɰ�������������ɼ�����ˮ��Ӧ�Ƶã���Ӧ���Ȼ�ѧ����ʽΪ��
CH4��g��+H2O

CO��g��+3H2��g������H=+QkJ/mol��Q��0��
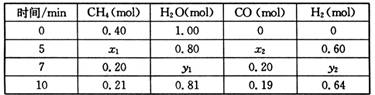
��3��һ���¶��£���2L�����з���������Ӧ�������ʵ����ʵ����仯���±�
�ٷ����������ݣ��ж�5?7min֮�䷴Ӧ�Ƿ���ƽ��״̬______����ǡ�����
ǰ5minƽ����Ӧ����v��CH4��=______��
�ڷ�Ӧ��7��10min֮�䣬CO�����ʵ������ٵ�ԭ�������______������ĸ����
a?����CH4 b?�����¶�c?����ѹǿd?����H2
���������¶Ȳ��䣬��1L��������ʼ����0.15mol CH4.0.45mol H2O��______mol CO��______mol H2���ﵽƽ��ʱCH4������ٷֺ������һ��Ͷ����ͬ��
��1����ϳ����а�1��4�����ʵ���֮�ȳ���N2��H2���а��ĺϳɣ�ͼAΪT0Cʱƽ�������а��������������ѹǿ��P���Ĺ�ϵͼ��

��д����ҵ�Ϻϳɰ��Ļ�ѧ����ʽ______��
��ͼA�а������������Ϊ15.00%ʱ��H2��ת����=______��
��ͼB��T=500��C���¶�Ϊ4500C��Ӧ��������______��ѡ����ĸ��a����b������ѡ���������______
����ͼ��֪������ѹǿ�����ԭ�ϵ������ʣ�������ʵ�ʿ�������ѹǿ������������______��д��һ�����ɣ���
��2���ϳɰ�������������ɼ�����ˮ��Ӧ�Ƶã���Ӧ���Ȼ�ѧ����ʽΪ��
CH4��g��+H2O

CO��g��+3H2��g������H=+QkJ/mol��Q��0��
��3��һ���¶��£���2L�����з���������Ӧ�������ʵ����ʵ����仯���±�
| ʱ��/min | CH4��mol�� | H20�� mol�� | CO ��mol�� | H2 ��mol�� |
| 0 | 0.40 | 1.00 | 0 | 0 |
| 5 | X1 | X2 | X3 | 0.60 |
| 7 | Y1 | Y2 | 0.20 | Y3 |
| 10 | 0.21 | 0.81 | 0.19 | 0.62 |
ǰ5minƽ����Ӧ����v��CH4��=______��
�ڷ�Ӧ��7��10min֮�䣬CO�����ʵ������ٵ�ԭ�������______������ĸ����
a?����CH4 b?�����¶�c?����ѹǿd?����H2
���������¶Ȳ��䣬��1L��������ʼ����0.15mol CH4.0.45mol H2O��______mol CO��______mol H2���ﵽƽ��ʱCH4������ٷֺ������һ��Ͷ����ͬ��
��1���ٹ�ҵ�����õ������������ϳɰ���ԭ������ʽΪ��N2+3H2
2NH3���ʴ�Ϊ��N2+3H2
2NH3��
����N2��ת����Ϊx����N2+3H2
2NH3
��ʼ���ʵ�����1 4 0
�仯�����ʵ�����x 3x 2x
ƽ��ʱ�����ʵ�����1-x 4-3x 2x
�������������Ϊ15.00%ʱ����
=15%
���x=0.33��mol��������������ת����=
��100%��24.45%���ʴ�Ϊ��24.45%��
�۶��ڷ�ӦN2+3H2
2NH3�����¶Ƚ���ʱ����ѧƽ������ȷ�Ӧ�����ƶ����������������������Ӧ��������b��
�ʴ�Ϊ��b���ϳɰ�������Ӧ�Ƿ��ȷ�Ӧ��������������ԭ���������������䣬�����¶ȣ���ѧƽ������ȷ�Ӧ�����ƶ���
������ѹǿ�����ԭ�ϵ������ʣ������豸����ѹ�̶������ģ�������ʵ�ʿ�������ѹǿ����������ѹ�豸������ѹǿ�Ķ��������ϵ�ǿ�ȵȣ�
�ʴ�Ϊ��������ѹ�豸��������ѹǿ�Ķ��������ϵ�ǿ�ȵ����������𰸣���
��2���ٸ���һ����̼�����������ʵ����ı仯��������жϣ�5?7min֮�䷴Ӧ�Ǵ���ƽ��״̬��ǰ5minƽ����Ӧ����v��CH4��=
=
=0.02mol?min-1��
�ʴ�Ϊ���ǣ�0.02mol?min-1��
�ڷ�Ӧ��7��10min֮�䣬CO�����ʵ������٣�������������ƽ�������ƶ�������һ����̼��Ũ�ȣ��ʴ�Ϊ��d��
���������¶Ȳ��䣬��1L��������ʼ����0.15molCH4��0.45molH2O���ٳ���0.05molCO��0.15molH2�൱����0.05mol�ļ����0.05mol��ˮ�������������0.2mol��ˮ��0.5mol���ͳ�ʼͶ��0.4mol�ļ����1.0mol��ˮ�ǵ�Ч�ģ��ﵽƽ��ʱCH4������ٷֺ�����ͬ���ʴ�Ϊ��0.05��0.15��
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
����N2��ת����Ϊx����N2+3H2
| ||
| ���¸�ѹ |
��ʼ���ʵ�����1 4 0
�仯�����ʵ�����x 3x 2x
ƽ��ʱ�����ʵ�����1-x 4-3x 2x
�������������Ϊ15.00%ʱ����
| 2x |
| 2x+1-x+4-3x |
���x=0.33��mol��������������ת����=
| 0.33��3 |
| 4 |
�۶��ڷ�ӦN2+3H2
| ||
| ���¸�ѹ |
�ʴ�Ϊ��b���ϳɰ�������Ӧ�Ƿ��ȷ�Ӧ��������������ԭ���������������䣬�����¶ȣ���ѧƽ������ȷ�Ӧ�����ƶ���
������ѹǿ�����ԭ�ϵ������ʣ������豸����ѹ�̶������ģ�������ʵ�ʿ�������ѹǿ����������ѹ�豸������ѹǿ�Ķ��������ϵ�ǿ�ȵȣ�
�ʴ�Ϊ��������ѹ�豸��������ѹǿ�Ķ��������ϵ�ǿ�ȵ����������𰸣���
��2���ٸ���һ����̼�����������ʵ����ı仯��������жϣ�5?7min֮�䷴Ӧ�Ǵ���ƽ��״̬��ǰ5minƽ����Ӧ����v��CH4��=
| ��c |
| ��t |
| ||
| 5min |
�ʴ�Ϊ���ǣ�0.02mol?min-1��
�ڷ�Ӧ��7��10min֮�䣬CO�����ʵ������٣�������������ƽ�������ƶ�������һ����̼��Ũ�ȣ��ʴ�Ϊ��d��
���������¶Ȳ��䣬��1L��������ʼ����0.15molCH4��0.45molH2O���ٳ���0.05molCO��0.15molH2�൱����0.05mol�ļ����0.05mol��ˮ�������������0.2mol��ˮ��0.5mol���ͳ�ʼͶ��0.4mol�ļ����1.0mol��ˮ�ǵ�Ч�ģ��ﵽƽ��ʱCH4������ٷֺ�����ͬ���ʴ�Ϊ��0.05��0.15��

��ϰ��ϵ�д�
�����Ŀ

 CO��g��+3H2��g������H=+QkJ/mol��Q��0��
CO��g��+3H2��g������H=+QkJ/mol��Q��0��
 2NH3��g������H=-92.4kJ/mol
2NH3��g������H=-92.4kJ/mol CO(g)+3H2(g) ��H>0��һ���¶��£������Ϊ2 L�ĺ��������з���������Ӧ�������ʵ����ʵ����仯���±���
CO(g)+3H2(g) ��H>0��һ���¶��£������Ϊ2 L�ĺ��������з���������Ӧ�������ʵ����ʵ����仯���±���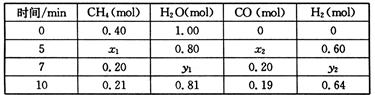
 CO(g)+3H2(g)
��H>0��һ���¶��£������Ϊ2 L�ĺ��������з���������Ӧ�������ʵ����ʵ����仯���±���
CO(g)+3H2(g)
��H>0��һ���¶��£������Ϊ2 L�ĺ��������з���������Ӧ�������ʵ����ʵ����仯���±���