��Ŀ����
4����Դ�ǹ��÷�չ����Ҫ�������ҹ�Ŀǰʹ�õ���Դ��Ҫ�ǻ�ʯȼ�ϣ���1����25�桢101kPaʱ��16g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31kJ����CH4ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.31kJ•mol-1 ��
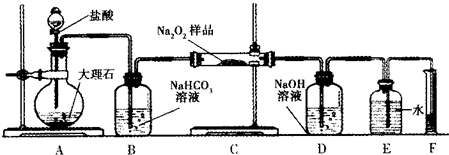
��2����ͼ��ʾ��ɱպϻ�·�����У���װ����CH4Ϊ������O2��CO2�Ļ������Ϊ������ϡ����������Ϊ�缫��������̼����Ϊ����ʣ���װ����a��bΪʯī��b�����к�ɫ����������CuSO4��Һ�����Ϊ200mL��
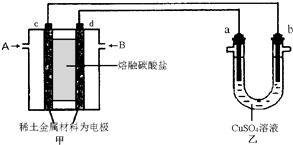
�ټ�װ��������AΪCH4���CH4����O2��CO2������d���ϵĵ缫��ӦʽΪO2+4e-+2CO2=2CO32-��
����װ����a���ϵĵ缫��ӦʽΪ4OH--4e-=O2��+2H2O��
����a������112mL����״�������壬���װ��������CH456 mL ����״��������װ����������Һ��pH=1�������Ե��ǰ����Һ����仯��
��������е缫���䣬����Һ���ɱ���Na2SO4��Һ������������a mol��������ʱ��ͬʱ��w g Na2SO4•10H2O�������������¶Ȳ��䣬ʣ����Һ�����ʵ���������ӦΪ$\frac{142w}{322��w+18a��}��100%$���ú�w��a�ı���ʽ��ʾ�����ػ���
���� ��1��16g��������ʵ�����1mol��16g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31kJ����1molCH4��ȫȼ������Һ̬ˮʱ�ų���������890.31kJ���ݴ���д�Ȼ�ѧ����ʽ��
��2����b�缫���к�ɫ�������ɣ���b������������a��������c�Ǹ�����d��������ͨ�����ĵ缫�Ǹ���������A�Ǽ��顢B�Ƕ�����̼��������d�缫�������õ��ӺͶ�����̼��Ӧ����̼������ӣ�
��a�����������������������ӷŵ�����������
����ת�Ƶ�����ȼ������ļ������������������������֮��Ĺ�ϵʽ�������������ʵ���Ũ�ȣ��Ӷ�ȷ��pH��
�۵�ⱥ����������Һʱ���������������������������������������൱�ڵ��ˮ�����������������������ʵ���֮����2��1�����Ե���������a mol��������ʱ�������������������ʵ���Ϊ0.5amol�����ˮ������=amol��2g/mol+0.5amol��32g/mol=18ag��
ʣ����Һ��Ȼ�DZ�����Һ������m��Na2SO4��=wg��$\frac{142}{322}$��
��Һ��������=$\frac{wg��\frac{142}{233}}{wg+18ag}��100%$��
��� �⣺��1��16g��������ʵ�����1mol��16g CH4��ȫȼ������Һ̬ˮʱ�ų���������890.31kJ����1molCH4��ȫȼ������Һ̬ˮʱ�ų���������890.31kJ�����Լ����ȼ���Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.31kJ•mol-1 ��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.31kJ•mol-1 ��
��2����b�缫���к�ɫ�������ɣ���b������������a��������c�Ǹ�����d��������ͨ�����ĵ缫�Ǹ���������A��CH4��B�Ƕ�����̼��������d�缫�������õ��ӺͶ�����̼��Ӧ����̼������ӣ��缫��ӦʽΪO2+4e-+2CO2=2CO32-��
�ʴ�Ϊ��CH4��O2+4e-+2CO2=2CO32-��
��a�����������������������ӷŵ������������缫��ӦʽΪ4OH--4e-=O2��+2H2O��
a��������n��O2��=$\frac{0.112L}{22.4L/mol}$=0.005mol������0.005mol����ת�Ƶ������ʵ���=0.005mol��4=0.02mol������ת�Ƶ�����ȼ������ļ������=$\frac{0.02mol}{8}��22.4L/mol$=56mL��
��ط�ӦʽΪ2Cu2++2H2O=4 H++O2��+2Cu�����ݷ���ʽ֪��n�� H+��=4n��O2��=4��0.005mol=0.02mol��c��H+��=$\frac{0.02mol}{0.2L}$=0.1mol/L������Һ��pH=1��
�ʴ�Ϊ��4OH--4e-=O2��+2H2O��56��1��
�۵�ⱥ����������Һʱ���������������������������������������൱�ڵ��ˮ�����������������������ʵ���֮����2��1�����Ե���������a mol��������ʱ�������������������ʵ���Ϊ0.5amol�����ˮ������=amol��2g/mol+0.5amol��32g/mol=18ag��
ʣ����Һ��Ȼ�DZ�����Һ������m��Na2SO4��=wg��$\frac{142}{322}$��
��Һ��������=$\frac{wg��\frac{142}{322}}{wg��18ag}��100%$=$\frac{142w}{322��w+18a��}��100%$��
�ʴ�Ϊ��$\frac{142w}{322��w+18a��}��100%$��
���� ���⿼���Ȼ�ѧ��Ӧ����ʽ����д��ԭ��غ͵���ԭ�������������йؼ��㣬���ؿ���ѧ������������������ȷ�����缫�Ϸ����ķ�Ӧ���ת�Ƶ�����Ƚ����йؼ��㣬�ѵ��ǣ�2���ۼ��㣬����������������ˮ������Һ�����������ɣ���Ŀ�ѶȲ���

| A�� | v��H2��=0.03 mol/��L•min�� | B�� | v��N2��=0.02 mol/��L•min�� | ||
| C�� | v��NH3��=0.01 mol/��L•min�� | D�� | v��NH3��=0.17 mol/��L•min�� |
| A�� | �ı�ijƽ����ϵ���¶� | |
| B�� | ����̬���ʲμӵĿ��淴Ӧ�ﵽƽ��ı�ѹǿ | |
| C�� | ʹƽ�������и���ֵ�Ũ�ȷ����仯 | |
| D�� | ���淴Ӧ�ﵽƽ���ʹ�ô��� |
| A�� | �����¶Ƚ����̷�Ӧ�ﵽƽ���ʱ�� | |
| B�� | ����Ӧ��ϵ��ѹǿ��Ӧ����һ������ | |
| C�� | ����V2O5ͬʱ�ı����淴Ӧ���� | |
| D�� | ����O2��Ũ�Ƚ����SO2��ת���� |
| A�� | ���ǵĻ�ѧ������ȫ��ͬ | B�� | ������̼Ԫ�ص�����ͬλ�� | ||
| C�� | ������̼Ԫ�ص����ֵ��� | D�� | ������̼Ԫ�ص�ͬ�������� |

 ��ϩ�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��������Ϊ��ϩ�ܹ�ת��Ϊ������Ҫ���л�����ԭ�ϣ����磺
��ϩ�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ��������Ϊ��ϩ�ܹ�ת��Ϊ������Ҫ���л�����ԭ�ϣ����磺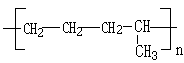 ��
�� ��
�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O��