��Ŀ����
9��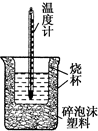 50mL 0.5mol•L-1������50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.5mol•L-1������50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������ǻ��β����������
��2�����ձ����粻��Ӳֽ�壬��õ��к���ƫ���ƫ��ƫС������Ӱ�족����
��3��ʵ���и���60mL 0.50mol•L-1�����50mL 0.50mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ��ƫ����ȡ���ƫС�����������к�����ȣ����ȡ�����ȡ������������ɣ����к�����ָϡǿ����ϡǿ����кͷ�Ӧ����1molH2O�ų��������������������أ�
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��ƫС����50mL 0.5mol•L-1������50mL 0.50mol•L-1 NaOH��Һ�����кͲⶨ����õ��к�����ֵ����Ӱ�죮���ƫ����ƫС������Ӱ�족��
��5��ȡ50mL 0.50mol/L NaOH��Һ��30mL0.50mol/L������Һ����ʵ�飬ʵ���������±���
| �¶� ʵ����� | ��ʼ�¶�T1/�� | ��ֹ�¶�T2/�� | �¶Ȳ�ƽ��ֵ��T2-T1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 30.1 | ||
| 2 | 27.0 | 27.4 | 33.3 | ||
| 3 | 25.9 | 25.9 | 29.8 | ||
| 4 | 26.4 | 26.2 | 30.4 | ||
��������NaOH��Һ��������Һ���ܶȾ�ȡ1g/mL����Һ�ı����ݾ�ȡ4.2J/��g���棩��
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2������Ӳֽ�壬����һ��������ɢʧ��
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��4��NH3•H2O��������ʣ��������ȣ������к��ȵĸ����ʵ�����ش�
��5���ȸ��ݱ��вⶨ���ݼ�������Һ��Ӧǰ���ƽ���¶Ȳ�ٸ���Q=cm��T�������Ӧ�ų���������Ȼ����������1molˮ�ų����������Ϳ��Եõ��к��ȣ�
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
��2�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ�����к���Ϊ��ֵ�����Բ�õ��к���ƫ��
�ʴ�Ϊ��ƫ��
��3����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL 0.50mol•L-1�����50mL 0.50mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ����Ը���60mL 0.50mol•L-1�����50mL 0.50mol•L-1 NaOH��Һ��������ʵ�飬����к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ����к�����ָϡǿ����ϡǿ����кͷ�Ӧ����1molH2O�ų��������������������أ�
��4����ˮΪ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС���к�����ֵ��ƫС���к�����ǿ���ǿ�Ӧ����1molˮʱ�ų��������������������أ�������50mL 0.5mol•L-1������50mL 0.50mol•L-1 NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��Ӱ�죻
�ʴ�Ϊ��ƫС����Ӱ�죻
��5����1��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ26.1�棬��Ӧ���¶�Ϊ30.1�棬��Ӧǰ���¶Ȳ�Ϊ4.0�棻
��2��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ27.2�棬��Ӧ���¶�Ϊ33.3�棬��Ӧǰ���¶Ȳ�Ϊ��6.1�棻
��3��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ25.9�棬��Ӧ���¶�Ϊ29.8�棬��Ӧǰ���¶Ȳ�Ϊ��3.9�棻
��4��ʵ�������NaOH��Һ��ʼƽ���¶�Ϊ26.3�棬��Ӧ���¶�Ϊ30.4�棬��Ӧǰ���¶Ȳ�Ϊ��4.1�棻
��2���������ϴ���ȥ�����������ƽ��ֵΪ4.0�棻
ȡ50mL 0.50mol/L NaOH��Һ��30mL0.50mol/L������Һ����ʵ�飬����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ80mL��1g/cm3=80g���¶ȱ仯��ֵΪ��T=4.0�棬������0.025molˮ�ų�������ΪQ=m•c•��T=80g��4.2J/��g•�棩��4.0��=1344J����1.344KJ������ʵ���õ��к��ȡ�H=-$\frac{1.344KJ}{0.025mol}$=-53.8kJ/mol��
�ʴ�Ϊ��-53.8kJ/mol��
���� ���⿼���ȷ�Ӧ�ȵIJⶨ����㣬��Ŀ�Ѷȴ�ע������ⶨԭ���ǽ���Ĺؼ�����������������ѧ�����Ӧ����ѧ֪ʶ��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �ɲ�ͬԭ�ӹ��ɵĴ�����һ���ǻ����� | |
| B�� | �ǽ���������һ�������������� | |
| C�� | ������Ԫ�ص�����һ������������ | |
| D�� | �����Ǵ����ˮ���ǻ���� |
| A�� | ��ȼ | B�� | ͨ��������ȼ | C�� | NaOH��Һ | D�� | �����ȵ�����ͭ |
| A�� | Ca��ClO��2��ClԪ�ػ��ϼ�Ϊ-1�� | |
| B�� | Ca��OH��2�ڸ÷�Ӧ��ʧȥ���ӣ����ֳ���ԭ�� | |
| C�� | Cl2�������������ǻ�ԭ�� | |
| D�� | ����1molCl2�μӷ�Ӧ����ת��2mol���� |

��֪������ؽ������������������������pH���±�����ʼ������pH����������Ũ��Ϊ1.0mol•L-1���㣩��
| �������� | ��ʼ������pH | ������ȫ��pH |
| Fe3+ | 1.1 | 3.2 |
| Fe2+ | 5.8 | 8.8 |
| Al3+ | 3.0 | 5.0 |
| Ni2+ | 6.7 | 9.5 |
��2����������������ͬ���ڲ�ͬ�¶��¶Է����������С������������������ʱ��仯��ͼ2����������������¶���ʱ��ֱ�ΪC������ĸ��
a��30�桢30min������������ b��90�桢150min
c��70�桢120min���������� d��90�桢120min
��3���������еġ�����Һ���õ�����Һx���������Ǽ�����H2SO4��Һ���ټ�����H2SO4��Һ����ַ�Ӧ����NaOH��Һ����pH5.0��pH��6.7���Χ������ַ�Ӧ����ˣ��Գ�ȥ������Ԫ�أ�
��4����������������õ��Ļ������ˣ����ù������Ҵ�ϴ�ӡ�110���º�ɰ����ò��������壮
�����Ҵ�ϴ�ӵ�Ŀ����ϴȥ��NH4��2SO4���ʡ����ں�ɣ����ٲ�Ʒ��ʧ��
�ں���¶Ȳ�����110���ԭ���Ƿ�ֹ�¶ȹ��ߣ�����������ֽ��ʧȥ�ᾧˮ��
��2H2��g��+O2��g���T2H2O��g����H2����2H2��g��+O2��g���T2H2O��l����H3��
����ȡ�����Ϊ4��1�ļ��������11.2L���ѻ���ɱ�״����������ȫȼ�պ�ָ������£��ų�������Ϊ��������
| A�� | -��0.4 mol����H1+0.05 mol����H3�� | B�� | -��0.4 mol����H1+0.05 mol����H2�� | ||
| C�� | -��0.4 mol����H1+0.1 mol����H3�� | D�� | -��0.4 mol����H1+0.1 mol����H2�� |