��Ŀ����
14��Ư�۾�������ɱ�����ã���ҵ�ϲ�����������ʯ�ҷ�Ӧ�Ƶã���Ӧ����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H20�������йظ÷�Ӧ��˵����ȷ���� ��������| A�� | Ca��ClO��2��ClԪ�ػ��ϼ�Ϊ-1�� | |
| B�� | Ca��OH��2�ڸ÷�Ӧ��ʧȥ���ӣ����ֳ���ԭ�� | |
| C�� | Cl2�������������ǻ�ԭ�� | |
| D�� | ����1molCl2�μӷ�Ӧ����ת��2mol���� |
���� ��Ӧ2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H20��ֻ��ClԪ�ػ��ϼ۷����仯���ֱ���0�۱仯Ϊ-1�ۡ�+1�ۣ��Դ˽�����
��� �⣺A���ɻ��ϼ۴�����Ϊ0��֪Ca��ClO��2��ClԪ�ػ��ϼ�Ϊ+1�ۣ���A����
B��ֻ��ClԪ�ػ��ϼ۷����仯����B����
C��ֻ��ClԪ�ػ��ϼ۷����仯����Cl2�������������ǻ�ԭ������C��ȷ��
D��ClԪ�ػ��ϼ���0�۱仯Ϊ-1�ۡ�+1�ۣ�����1molCl2�μӷ�Ӧ����ת��1mol���ӣ���D����
��ѡC��
���� ���⿼��������ԭ��Ӧ��Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע��Ԫ�ػ��ϼ۵ı仯��Ϊ��������Ŀ�Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
�����Ŀ
4�� ��һ��þ���Ͻ�Ͷ�뵽1mol/L HCl��Һ����Ͻ���ȫ�ܽ��������Һ�����1mol/L NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ����仯�Ĺ�ϵ��ͼ��ʾ������˵���д�����ǣ�������
��һ��þ���Ͻ�Ͷ�뵽1mol/L HCl��Һ����Ͻ���ȫ�ܽ��������Һ�����1mol/L NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ����仯�Ĺ�ϵ��ͼ��ʾ������˵���д�����ǣ�������
 ��һ��þ���Ͻ�Ͷ�뵽1mol/L HCl��Һ����Ͻ���ȫ�ܽ��������Һ�����1mol/L NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ����仯�Ĺ�ϵ��ͼ��ʾ������˵���д�����ǣ�������
��һ��þ���Ͻ�Ͷ�뵽1mol/L HCl��Һ����Ͻ���ȫ�ܽ��������Һ�����1mol/L NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ����仯�Ĺ�ϵ��ͼ��ʾ������˵���д�����ǣ�������| A�� | HCl��Һ�����Ϊ80mL | B�� | a��ȡֵ��ΧΪ0��a��50 | ||
| C�� | ��aֵΪ30ʱ��bֵΪ0.01 | D�� | $\frac{n��Mg��}{n��Al��}$�����ֵΪ2.5 |
2������ȷ��ʾ���з�Ӧ�����ӷ���ʽ�ǣ�������
| A�� | ��ʯī���缫����Ȼ�þ��Һ��2H2O+2Cl-$\frac{\underline{\;���\;}}{\;}$-H2��+Cl2��+2OH- | |
| B�� | �ù�����ˮ���չ�ҵβ���е�SO2��2NH3•H20+SO2=2NH4++SO32-+H2O | |
| C�� | ��������������ϡ���3Fe2++4H++NO3-=3Fe3++NO��+3H2O | |
| D�� | ������Һ�е���Ba��OH��2��ҺʹSO42-ǡ����ȫ������ 2Ba2++3OH-+Al3++2SO42-=2BaSO4��+Al��OH��3�� |
9��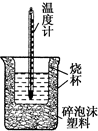 50mL 0.5mol•L-1������50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.5mol•L-1������50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������ǻ��β����������
��2�����ձ����粻��Ӳֽ�壬��õ��к���ƫ���ƫ��ƫС������Ӱ�족����
��3��ʵ���и���60mL 0.50mol•L-1�����50mL 0.50mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ��ƫ����ȡ���ƫС�����������к�����ȣ����ȡ�����ȡ������������ɣ����к�����ָϡǿ����ϡǿ����кͷ�Ӧ����1molH2O�ų��������������������أ�
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��ƫС����50mL 0.5mol•L-1������50mL 0.50mol•L-1 NaOH��Һ�����кͲⶨ����õ��к�����ֵ����Ӱ�죮���ƫ����ƫС������Ӱ�족��
��5��ȡ50mL 0.50mol/L NaOH��Һ��30mL0.50mol/L������Һ����ʵ�飬ʵ���������±���
���к��ȡ�H=-53.8kJ/mol��ȡС�����һλ����
��������NaOH��Һ��������Һ���ܶȾ�ȡ1g/mL����Һ�ı����ݾ�ȡ4.2J/��g���棩��
 50mL 0.5mol•L-1������50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.5mol•L-1������50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ��������ǻ��β����������
��2�����ձ����粻��Ӳֽ�壬��õ��к���ƫ���ƫ��ƫС������Ӱ�족����
��3��ʵ���и���60mL 0.50mol•L-1�����50mL 0.50mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�����������ȣ��ƫ����ȡ���ƫС�����������к�����ȣ����ȡ�����ȡ������������ɣ����к�����ָϡǿ����ϡǿ����кͷ�Ӧ����1molH2O�ų��������������������أ�
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��ƫС����50mL 0.5mol•L-1������50mL 0.50mol•L-1 NaOH��Һ�����кͲⶨ����õ��к�����ֵ����Ӱ�죮���ƫ����ƫС������Ӱ�족��
��5��ȡ50mL 0.50mol/L NaOH��Һ��30mL0.50mol/L������Һ����ʵ�飬ʵ���������±���
| �¶� ʵ����� | ��ʼ�¶�T1/�� | ��ֹ�¶�T2/�� | �¶Ȳ�ƽ��ֵ��T2-T1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 30.1 | ||
| 2 | 27.0 | 27.4 | 33.3 | ||
| 3 | 25.9 | 25.9 | 29.8 | ||
| 4 | 26.4 | 26.2 | 30.4 | ||
��������NaOH��Һ��������Һ���ܶȾ�ȡ1g/mL����Һ�ı����ݾ�ȡ4.2J/��g���棩��
19�����и����е�������Һ�����������ơ�����������ϡ���ᡢ����ʯ��ˮ�����Ȼ��ơ��������������ᡢ�������������ƣ����Ȼ������������ƣ���̼���ơ�ϡ���������������ˮ �������������Լ���ֻ��������ϼ��ɼ�����ǣ�������
| A�� | �ܢݢޢ� | B�� | �٢ڢۢܢݢ� | C�� | �٢ۢܢ� | D�� | ȫ�� |
6�����и����е����ӷ���ʽ����д��ȷ���� ��������
| A�� | AlCl3 ˮ�⣺Al3++3H2O�TAl��OH��3 +3H+ | |
| B�� | NaHCO3 ���룺NaHCO3 ?Na++HCO3- | |
| C�� | ����Ca��OH��2 ��NH4Cl���ȣ�NH4++OH-�TNH3+H2O | |
| D�� | ������CO2 ����ͨ�뱥��ʯ��ˮ�У�CO2 +OH-�THCO3- |
11����NA��ʾ�����ӵ�������ֵ��������������ȷ���ǣ�������
| A�� | 10 L 0��l mol•L-1��Na2CO3��Һ����������һ������3NA | |
| B�� | ��״���£�22.4 L HF�ķ�����ΪNA | |
| C�� | 1 mol Fe��22.4 L��Cl2����״���£���ȼ�գ�ת�Ƶĵ�������Ϊ3NA | |
| D�� | һ��������ij�ܱ�����ʢ��0.1 mol N2��0.3 mol H2����ַ�Ӧ��ת�Ƶ�����Ϊ0.6NA |
 ��B��Ԫ�����ڱ���λ�õڶ����ڵڢ�A��
��B��Ԫ�����ڱ���λ�õڶ����ڵڢ�A��