��Ŀ����
����Ŀ���������仯�����ڹ�ũҵ�����ж�����Ҫ���á�
��1����̬��ԭ�Ӽ۵����Ų��Ĺ����ʾʽΪ___________��
��2��Ԫ��B��N��O�ĵ�һ�������ɴ�С��˳��Ϊ___________��
��3��ʳƷ���Ӽ�NaNO2��NO2-����ԭ�ӵ��ӻ�������_____����NO2-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ___________��(д1��)��
��4��N2H4�ǻ����ȼ�ϣ�����������Է���������ͬ�����ڳ��³�ѹ����Һ̬������������̬��������ֲ������Ҫԭ����____________��
��5��������һ����ĥͿ�ϣ��������������ı��汣���㡣����������廯������廯���ڸ������������з�Ӧ�ϳɡ�
�����廯���ӵĿռ乹����_________�����廯�������___________��
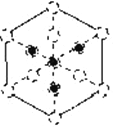
�������徧����ͼ��ʾ:����ʵ����Ϊ��ԭ�ӣ���һ����������ԭ�ӿռ�ѻ���ʽΪ________����ԭ�ӵ���λ��Ϊ___________���ýṹ����һ����λ�����ṩ�չ����ԭ����___________����֪�����߳�apm�������ӵ�����ΪNA������������ܶ�Ϊ___________g/cm3��

������������Խ��߷����ͶӰ(ͼ������ԲȦ��ʾPԭ�ӵ�ͶӰ)����ʵ��ԲȦ����Bԭ�ӵ�ͶӰλ��(ע��ԭ���������Դ�С)��______
���𰸡�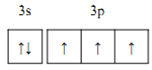 N>O>B sp2�ӻ� SO2��O3 N2H4���Ӽ���������O2���Ӽ�ֻ�з��»���������ȷ��»���ǿ ������ 120�� �����������ܶѻ� 4 B
N>O>B sp2�ӻ� SO2��O3 N2H4���Ӽ���������O2���Ӽ�ֻ�з��»���������ȷ��»���ǿ ������ 120�� �����������ܶѻ� 4 B ![]()
 ��
��
��������
(1)PΪ15��Ԫ�أ���̬��ԭ�Ӽ۵����Ų��Ĺ����ʾʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(2)ͬһ���ڣ������ң�Ԫ���ĵ�һ������������N��2pΪ�����״̬����Ϊ�ȶ�����һ�����ܴ���O����һ�������ɴ�С��˳��ΪN>O>B���ʴ�Ϊ��N>O>B��
(3)ʳƷ���Ӽ�NaNO2��NO2-����ԭ��N��2��ԭ���������¶Ե��Ӷ���=![]() (5+1-2��2)=1������sp2����NO2-��Ϊ�ȵ�����ķ�����SO2��O3���ʴ�Ϊ��sp2�ӻ���SO2��O3��
(5+1-2��2)=1������sp2����NO2-��Ϊ�ȵ�����ķ�����SO2��O3���ʴ�Ϊ��sp2�ӻ���SO2��O3��
(4)N2H4���Ӽ���������O2���Ӽ�ֻ�з��»���������ȷ��»���ǿ�������ڳ��³�ѹ��N2H4��Һ̬������������̬���ʴ�Ϊ��N2H4���Ӽ���������O2���Ӽ�ֻ�з��»���������ȷ��»���ǿ��
(5)�����廯��������ԭ�ӵļ۲���Ӷ���Ϊ![]() =4��Pԭ�Ӱ�sp3��ʽ�ӻ�����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ����廯���������ԭ�ӵļ۲���Ӷ���Ϊ
=4��Pԭ�Ӱ�sp3��ʽ�ӻ�����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ����廯���������ԭ�ӵļ۲���Ӷ���Ϊ![]() =3��Bԭ�Ӱ�sp2��ʽ�ӻ���û�йµ��Ӷԣ����Է��ӿռ乹��Ϊƽ�������Σ��ṹʽΪ
=3��Bԭ�Ӱ�sp2��ʽ�ӻ���û�йµ��Ӷԣ����Է��ӿռ乹��Ϊƽ�������Σ��ṹʽΪ![]() ��������120�����ʴ�Ϊ�������Σ�120�㣻
��������120�����ʴ�Ϊ�������Σ�120�㣻
�ڸ��������徧���ṹͼ����һ����������ԭ�ӿռ�ѻ���ʽΪ�����������ܶѻ�����������ԭ�Ӻ���ԭ�Ӷ���4����B����λ��Ϊ4����P����λ��ҲΪ4���ýṹ����һ����λ����Bԭ���������3�����ӣ��ṩ�չ����ԭ����B����������ԭ�Ӻ���ԭ�Ӷ���4���������ܶ�=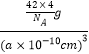 =
=![]() g/cm3���ʴ�Ϊ�������������ܶѻ���4��B��
g/cm3���ʴ�Ϊ�������������ܶѻ���4��B��![]() ��
��
�۸�������ľ����ṹͼ��������Խ��߷����ͶӰΪ (ͼ������ԲȦ��ʾPԭ�ӵ�ͶӰ��ʵ��ԲȦΪBԭ�ӵ�ͶӰ������Pԭ�Ӱ뾶��B�ʴ�Ϊ��
(ͼ������ԲȦ��ʾPԭ�ӵ�ͶӰ��ʵ��ԲȦΪBԭ�ӵ�ͶӰ������Pԭ�Ӱ뾶��B�ʴ�Ϊ�� ��
��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�����Ŀ����ҵ����﮻�ʯΪԭ������̼��﮵IJ��ֹ�ҵ�������£�

��֪����﮻�ʯ����Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2�����к�����FeO��MgO�ȡ�
��Li2O��Al2O3��4SiO2+H2SO4![]() Li2SO4+Al2O3��4SiO2��H2O
Li2SO4+Al2O3��4SiO2��H2O
��ijЩ���ʵ��ܽ��(S)���±���ʾ��
T�� | 20 | 40 | 60 | 80 |
S(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
S(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |
��Fe3+��ȫ����ʱpHΪ3.4
(1)Ϊ���ԭ�Ͻ������ʣ��������¶���ɲ�ȡ�Ĵ�ʩ��__________(��дһ��)��
(2)����Һ1�м���H2O2��Ŀ����__________(�����ӷ���ʽ��ʾ)������pH����Լ���__________(����� )��
A��CuO B��CuCO3 C��MgO D��NH3��H2O
(3)�������з����Al2O3����������ͼ��ʾ����д�����ɳ��������ӷ���ʽ__________��
![]()
(4)����Һ2�м���Na2CO3��Һ��������__________��
(5)�����ˡ���ˮϴ�ӡ������У�����ˮϴ�ӡ���ԭ����__________;֤��������ϴ���IJ�����__________��