��Ŀ����
����Ŀ��������AΪԭ�Ϻϳ����ָ߷��Ӳ��ϵ�·������ͼ��ʾ��
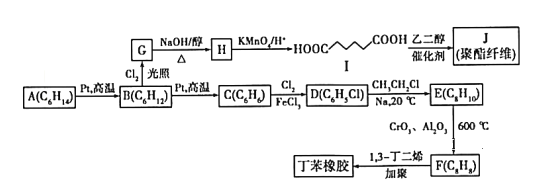
��֪������Ϣ���ٷ���ʽA(C6H14)��B(C6H12) ��C(C6H6) ��D(C6H5Cl) ��E(C8H10) ��F(C8H8)
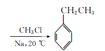
��B(C6H12)�ĺ˴Ź���������ֻ��1��壬GΪһ�ȴ�����
��R1-X+R2-X ![]() R1- R2 (X����±��ԭ�ӣ�R1��R2��������)��
R1- R2 (X����±��ԭ�ӣ�R1��R2��������)��
��![]()
![]()
![]() +R3-COOH(R1��R2��R3��������)��
+R3-COOH(R1��R2��R3��������)��
��ش��������⣺
(1) B�Ļ�ѧ����Ϊ______________________��
(2)��G����H�Ļ�ѧ����ʽΪ_____________________��
(3)��C����D�ķ�Ӧ����Ϊ_____________��
(4)F�Ľṹ��ʽΪ_________________��
(5)I��ͬ���칹������ͬʱ�������������Ĺ���____________��(���������칹)��
�����뱥��NaHCO3��Һ��Ӧ�������壻�ڼ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ�����к˴Ź�����������4��壬�ҷ����֮��Ϊ6��2��1��1����________(д������һ�ֵĽṹ��ʽ)��
(6)���������ϳ�·�ߣ���2-�������һ�ȼ���Ϊԭ��(���Լ���ѡ)������Ʊ�������E�ĺϳ�·��___________________��
���𰸡������� ![]() +NaOH
+NaOH![]() +NaCl+H2O ȡ����Ӧ
+NaCl+H2O ȡ����Ӧ 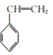 12
12 ![]()

��������
B(C6H12)�ĺ˴Ź���������ֻ��1��壬GΪһ�ȴ�������֪B�ǻ����飬G��һ�Ȼ����飬G���������ƵĴ���Һ�з�����ȥ��Ӧ���ɻ���ϩ��H��������ϩ����Ϊ![]() ��I�����������ڴ��������������ɷ���ʽΪC6H6���л���C��C�DZ��������������Ȼ����Ĵ������·���ȡ����Ӧ�����ȱ�(D)����R1-X+R2-X
��I�����������ڴ��������������ɷ���ʽΪC6H6���л���C��C�DZ��������������Ȼ����Ĵ������·���ȡ����Ӧ�����ȱ�(D)����R1-X+R2-X ![]() R1- R2����֪�ȱ��������鷴Ӧ����E����E���ұ����ұ��ڴ�������������F(C8H8)��F�DZ���ϩ��
R1- R2����֪�ȱ��������鷴Ӧ����E����E���ұ����ұ��ڴ�������������F(C8H8)��F�DZ���ϩ��
(1) B(C6H12)�ĺ˴Ź���������ֻ��1��壬B�Ľṹ��ʽ��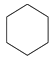 �������ǻ����顣
�������ǻ����顣
(2)G��һ�Ȼ����飬���������ƵĴ���Һ�з�����ȥ��Ӧ���ɻ���ϩ��H������ѧ����ʽΪ![]() +NaOH
+NaOH![]() +NaCl+H2O��
+NaCl+H2O��
(3)C�DZ��������������Ȼ����Ĵ������·���ȡ����Ӧ�����ȱ�(D)����Ӧ����Ϊȡ����Ӧ��
(4) F�DZ���ϩ��F�Ľṹ��ʽΪ ��
��
(5) �����뱥��NaHCO3��Һ��Ӧ�������壬˵�������Ȼ����ڼ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��˵���Ǽ�������I��ͬ���칹������ͬʱ������������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
�� ��
��![]() ��
�� ��
��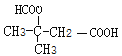 ��
��![]() ��
��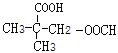 ����12��(���������칹)�����к˴Ź�����������4��壬�ҷ����֮��Ϊ6��2��1��1����
����12��(���������칹)�����к˴Ź�����������4��壬�ҷ����֮��Ϊ6��2��1��1����![]() ��
��
(6) 2-��������Pt�ڴ������ڸ������������ɼ������飬����������Pt���������ڸ������������ɼױ����ױ��ڹ�������������������ȡ����Ӧ����![]() ��
��![]() ��һ�ȼ��鷴Ӧ�����ұ����ϳ�·��Ϊ
��һ�ȼ��鷴Ӧ�����ұ����ϳ�·��Ϊ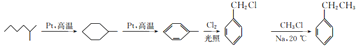 ��
��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ��ijͬѧ�������ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ���������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
���� | Al | Al2O3 | Fe | Fe2O3 |
�۵�/�� | 660 | 2054 | 1535 | 1462 |
�е�/�� | 2467 | 2980 | 2750 | -- |
I.��ͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ��������һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���_______����Ӧ�����ӷ���ʽΪ____________________________��
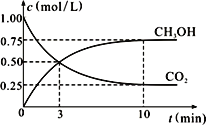
��. ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
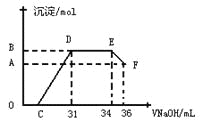
��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ������Ϊ��___________________��
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ�Ϊ_________��
��3����c=13mLʱ��ԭ��Һ��Fe3+��Al3+�����ʵ���֮��Ϊ________________________��