��Ŀ����
����Ŀ����1��ij����Һ����Na+��Fe2+��Ba2+��Al3+��NO3-��Cl-��SO42-�е�4�����ӣ��������Ӿ�Ϊ1mol���������Һ�м��������ϡ���ᣬ�����ݲ���������Һ������������䣨������ˮ�ĵ�������ӵ�ˮ�⣩���ش��������⣺
����Һ�д��ڵ�������______��
��д����Һ�м��������ϡ���ᷴӦ�����ӷ���ʽ______��
��2)��TiO2Ϊ������NaClO��CN������������CNO����CNO�������������¼�����NaClO��Ӧ����N2��CO2��Cl2�ȡ�ȡŨ����CN�����ӵķ�ˮ�����NaClO��Һ�Ļ��Һ��200mL��������CN����Ũ��Ϊ0.2mol��L��1������ʵ�顣
��д��CNO�������������±�NaClO���������ӷ���ʽ��________��
����������CO2������Ϊ1.408g�����ʵ���в��CN���������İٷ���Ϊ____��
��3������KMnO4��H2O2��NaClO���������������ҽ���г�����������������H2O2��������Ư�ף��ǻ�ѧʵ������ر�����Ҫ�����Լ������������ɵ����տ��û�ԭ�ԵIJ��� (H2C2O4)ȥ����Fe(NO3)3Ҳ����Ҫ�����Լ��������Ƕ����������������ʵ�̽����
�������ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬��Ӧ�Ļ�ѧ����ʽ________________��
��ȡ300 mL 0.2 mol/L��KI��Һ��һ����������KMnO4��Һǡ�÷�Ӧ�����ɵ����ʵ�����I2��KIO3��������KMnO4�����ʵ�������________mol��
����Fe(NO3)3��Һ�м���Na2SO3��Һ����Һ�����ػ�ɫ��Ϊdz��ɫ����һ���ֱ�Ϊ�ػ�ɫ��д����Һ�ȱ�Ϊdz��ɫ�����ӷ���ʽ��_________________________________��
���𰸡� Na+��Fe2+��NO3-��SO42- 3Fe2++NO3-+4H+=3Fe3++NO��+2H2O 2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O 80% Cu+2HCl+H2O2=CuCl2+2H2O 0.032mol 2Fe3++SO32-+H2O=2Fe2++SO42-+2H+
����������1���ټ��������ϡ���ᣬ�����ݲ���������Һ������������䣬����ֻ��ΪNO��ΪFe2+��NO3-֮��������ԭ��Ӧ���ɵģ���������������䣬��ԭ��Һ��һ������SO42-������Һ�к����������ӣ��������ӵ����ʵ�����Ϊ1mol�����ݵ���غ㣬һ�������д�һ����λ����ɵ������ӣ���һ������Na+����һ�����ڵ�������Na+��Fe2+��NO3-��SO42-��
����Һ�м��������ϡ���ᷴӦ�����ӷ���ʽΪ3Fe2++NO3-+4H+=3Fe3++NO��+2H2O����2)��CNO�������������±�NaClO���������ӷ���ʽΪ��2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O���� 1.408g CO2�����ʵ���Ϊ![]() �����ݷ�Ӧ2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O��CNO�����ʵ���Ϊ
�����ݷ�Ӧ2CNO-+6ClO-+8H+�TN2��+2CO2��+3Cl2��+4H2O��CNO�����ʵ���Ϊ![]() ������̼ԭ���غ㣬CN��������CNO-���ʵ������Ϊ
������̼ԭ���غ㣬CN��������CNO-���ʵ������Ϊ![]() ����ʼ��Һ��CN���������ʵ���=0.2
����ʼ��Һ��CN���������ʵ���=0.2![]() ����CN���������İٷ���Ϊ
����CN���������İٷ���Ϊ![]() ����3���������ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬��Ӧ�Ļ�ѧ����ʽCu+2HCl+H2O2=CuCl2+2H2O����0.300 L
����3���������ͭƬ��ϡ�����м���H2O2��ͭƬ�ܽ⣬��Ӧ�Ļ�ѧ����ʽCu+2HCl+H2O2=CuCl2+2H2O����0.300 L ![]() 0.2 mol/L=0.6molI-
0.2 mol/L=0.6molI-![]() 0.02molI2+0.02molIO3-��ת��0.16mol���ӣ���MnO4-
0.02molI2+0.02molIO3-��ת��0.16mol���ӣ���MnO4-![]() Mn2+���Կ��������ϼ���+7��Ϊ+2�ۣ��ɵ����غ��֪�μӷ�Ӧ��KMnO4�����ʵ�������
Mn2+���Կ��������ϼ���+7��Ϊ+2�ۣ��ɵ����غ��֪�μӷ�Ӧ��KMnO4�����ʵ�������![]() mol���������ػ�ɫ��Ϊdz��ɫ����һ���ֱ�Ϊ�ػ�ɫ����Fe2+�ȱ���ԭ�����������ȱ�Ϊdz��ɫ�����ӷ���ʽΪ��2Fe3++SO32-+H2O=2Fe2++SO42-+2H+��
mol���������ػ�ɫ��Ϊdz��ɫ����һ���ֱ�Ϊ�ػ�ɫ����Fe2+�ȱ���ԭ�����������ȱ�Ϊdz��ɫ�����ӷ���ʽΪ��2Fe3++SO32-+H2O=2Fe2++SO42-+2H+��

����Ŀ��ij�����ijɷ�ΪCu2O��Al2O3��Fe2O3��SiO2����ҵ���øÿ�����ȡͭ�͵����IJ����������£�
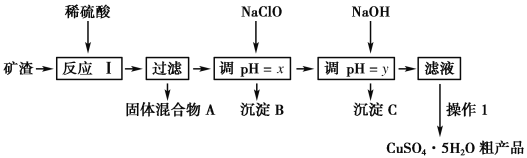
��֪����Cu2O��2H��===Cu��Cu2����H2O��
�ڲ���������������������ʽ����ʱ��Һ��pH���±���ʾ��
������ | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 |
��ʼ����pH | 5.4 | 4.0 | 2.7 | 5.8 |
������ȫpH | 6.7 | 5.2 | 3.7 | 8.8 |
��1��Ϊ�˼ӿ췴Ӧ������ʣ����Բ�ȡ�Ĵ�ʩ��____________ (д����)��
��2����������A�еijɷ���________��
��3����Ӧ����ɺ���Ԫ�صĴ�����ʽΪ________(�����ӷ���)��д�����ɸ����ӵ����ӷ���ʽ____________��
��4������1��Ҫ����������Ũ������ȴ�ᾧ��________��ϴ��CuSO4��5H2O�ֲ�Ʒ�����ô���ˮϴ�����ñ�ˮϴ�ӡ�ԭ����______________��
��5����NaCl0��pH�������ɳ���B����������������Ϣ��������BΪ_______���÷�Ӧ�����ӷ���ʽΪ______________��
����Ŀ������������������;�Ķ�Ӧ��ϵ����ȷ���ǣ� ��
ѡ�� | ���� | ��; |
A�� | ��������ǿ������ | ����Ư�� |
B�� | �������������ᷴӦ | ��������ҩ |
C�� | ����������ˮ | ������ˮ�� |
D�� | ���������� | ���ڽ����ӹ�ǰ����ϴ |
A. A B. B C. C D. D

