��Ŀ����
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ������һ�ַ�Ӧԭ�����£�
CH3OH(g) + H2O(g) �� CO2(g) + 3H2(g) �C 49.0 kJ
����˵����ȷ����
CH3OH(g) + H2O(g) �� CO2(g) + 3H2(g) �C 49.0 kJ
����˵����ȷ����
| A��1 LCH3OH������1 Lˮ������Ӧ����1 L CO2������3 L������������49.0 kJ |
| B��1��CH3OH������1��ˮ���ӷ�Ӧ����1��CO2������3��H2��������49.0 kJ���� |
| C����ͬ������1molCH3OH(g)��1mol H2O(g)�������ܺ�С��1molCO2(g)��3 mol H2(g)�������ܺ� |
| D��1 molCH3OH������1 molҺ̬ˮ��Ӧ����1mol CO2������3 mol �������յ�����С��49.0 kJ |
���������A��1molCH3OH������1molˮ������Ӧ����1mol CO2������3mol ������������49.0kJ����A����B��1molCH3OH������1molˮ������Ӧ����1mol CO2������3mol ������������49.0kJ����B����C�����ȷ�Ӧ�з�Ӧ��������������������������������1molCH3OH��g����1mol H2O��g���������ܺ�С��1molCO2��g����3 mol H2��g���������ܺͣ���C��ȷ��D��Һ̬ˮ����������Ϊ��̬������1 molCH3OH������1 molҺ̬ˮ��Ӧ����1mol CO2������3 mol �������յ���������49.0 kJ����D����ѡ��C��

��ϰ��ϵ�д�
�����Ŀ
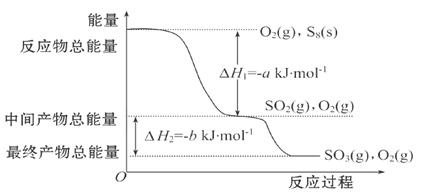
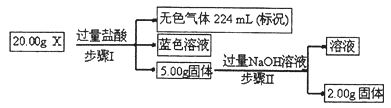
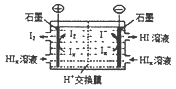
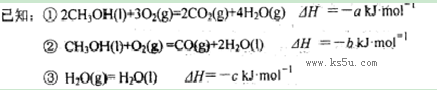
 CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ����K�����¶ȣ�T / �棩��ϵ���£�
CO(NH2)2 (l) + H2O (l)���÷�Ӧ��ƽ�ⳣ����K�����¶ȣ�T / �棩��ϵ���£� ����ͼ��1���ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ��1���ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��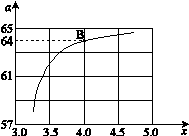
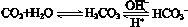 ��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±���
��ʹ����ѪҺpH������7.35��7.45������ͻᷢ�����ж�����ж�����pH��c(HCO3-)��c(H2CO3)�仯��ϵ���±��� 2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol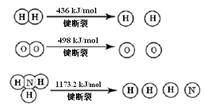
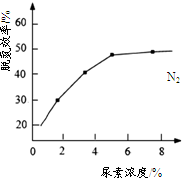
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%��
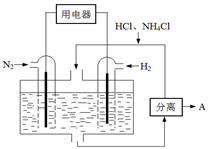
 CO2(g)��H2(g)���õ������������ݣ�
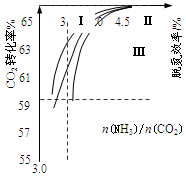
CO2(g)��H2(g)���õ������������ݣ� CO(NH2)2(l)��H2O(g)�ںϳ����н��С���ͼ�Т���������Ϊ�ϳ����а���ͬ��̼�� [n(NH3)/n(CO2)]��ˮ̼��[n(H2O)/n(CO2)]Ͷ��ʱ������̼ת���ʵ������
CO(NH2)2(l)��H2O(g)�ںϳ����н��С���ͼ�Т���������Ϊ�ϳ����а���ͬ��̼�� [n(NH3)/n(CO2)]��ˮ̼��[n(H2O)/n(CO2)]Ͷ��ʱ������̼ת���ʵ������