��Ŀ����
����Ŀ��(1)�����м������ʣ�A. ![]() B. ����ͼ��� C. ��������춡�� D. CH3CH3��CH3(CH2)8CH3
B. ����ͼ��� C. ��������춡�� D. CH3CH3��CH3(CH2)8CH3
E.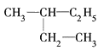 ��
��![]() F.
F.![]() ��
��![]()
���У�������ͬ���칹�����________��������ͬλ�ص���________��������ͬһ�����ʵ���________��
������ͬϵ�����________________��
(2)��֪ij����������Է�������Ϊ72��
�ٸ������ķ���ʽΪ______________��
��д���������������е�ͬ���칹��Ľṹ��ʽ____________________________��
������ͬ���칹���У�����ͬ�����·е���͵���_____________________________(д�ṹ��ʽ)��
(3) ��֪![]() �ı����ϵĶ���ȡ������6�֣���ױ������ϵ�����ȡ������______�֣�
�ı����ϵĶ���ȡ������6�֣���ױ������ϵ�����ȡ������______�֣�
(4)���и��ִ����ܷ�������������_______ ��
A. CH3OH B. CH3CH2OH C. CH3CH(CH3)OH D. CH3CH2C(CH3)2OH
���𰸡�CF A E BD C5H12 CH3CH2CH2CH2CH3��![]() ��
��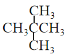
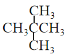 6 D
6 D
��������
��1������ʽ��ͬ�ṹ��ͬ�Ļ����ﻥΪͬ���칹�壬��������춡�顢![]() ��
��![]() �ֱ��������ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬��ѡCF����������ͬ��������ͬ��ͬһ��Ԫ�صIJ�ͬ���ػ�Ϊͬ���칹�壬��Ϊͬλ�ص���
�ֱ��������ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壬��ѡCF����������ͬ��������ͬ��ͬһ��Ԫ�صIJ�ͬ���ػ�Ϊͬ���칹�壬��Ϊͬλ�ص���![]() ����ѡA�����ʺͽṹ����ȫ��ͬ����ͬһ�����ʣ���
����ѡA�����ʺͽṹ����ȫ��ͬ����ͬһ�����ʣ���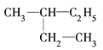 ��
��![]() ����ͬһ�����ʣ���ѡE���ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ���л������Ϊͬϵ������ͼ��顢CH3CH3��CH3(CH2)8CH3�ֱ�Ϊͬϵ���ѡBD��
����ͬһ�����ʣ���ѡE���ṹ���ƣ��������������ɸ�CH2ԭ���ŵ�ͬһ���л������Ϊͬϵ������ͼ��顢CH3CH3��CH3(CH2)8CH3�ֱ�Ϊͬϵ���ѡBD��
��2������֪ij����������Է�������Ϊ72����������ͨʽCnH2n+2��֪14n+2��72�����n��5�����Ը������ķ���ʽΪC5H12��
�ڸ����������飬��3��ͬ���칹�壬�������顢������������飬�����е�ͬ���칹��Ľṹ��ʽΪCH3CH2CH2CH2CH3��![]() ��
��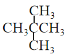 ��
��
������ͬ���칹���У�����ͬ�����·е���͵��Ǻ���֧�����ģ���Ϊ�����飬�ṹ��ʽΪ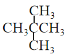 ��
��
��3����֪![]() �ı����ϵĶ���ȡ������6�֣����ڱ�����һ����5����ԭ�ӣ���ױ������ϵ�����ȡ����Ҳ��6�֣�
�ı����ϵĶ���ȡ������6�֣����ڱ�����һ����5����ԭ�ӣ���ױ������ϵ�����ȡ����Ҳ��6�֣�
��4��������������Ӧ�Ľṹ�ص��ǣ�ֻ���ǻ�����̼��̼������ԭ�ӵIJ��ܷ���������Ӧ�����CH3OH��CH3CH2OH��CH3CH(CH3)OH���ܷ�����������CH3CH2C(CH3)2OH���ܷ�������������ѡD��

����Ŀ����һ���������з�Ӧ:2SO2(g)+O2(g)![]() 2SO3(g) ��H=-197kJ/mol.�����ݻ���ͬ�ļס��ҡ����������������������������·ֱ���������ͷ�Ӧ�ų�������(Q)�����ʾ:
2SO3(g) ��H=-197kJ/mol.�����ݻ���ͬ�ļס��ҡ����������������������������·ֱ���������ͷ�Ӧ�ų�������(Q)�����ʾ:
���� | SO2(mol) | O2(mol) | N2(mol) | Q(kJ) |
�� | 2 | 1 | 0 | Q1 |
�� | 1 | 0.5 | 0 | Q2 |
�� | 1 | 0.5 | 1 | Q3 |
�����������ݣ�������������ȷ����:
A. Q1<197
B. �����������£���Ӧ����1molS03�������98.5kJ
C. Q2=Q3
D. Q3<Q1<2Q2
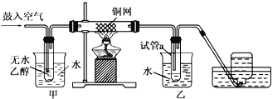
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50 mL 0.25 mol��L��1���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol��L��1NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ����¶ȡ�
�ش��������⣺
��1������NaOH��Һ����ȷ������__________��
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
��2��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������________��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β������������ؽ���
��3�������Ϊ�β���ͭ�ʵ�_____________.
��4��ʵ���������±���
������д�±��еĿհף�________��
�¶� ʵ����� | ��ʼ�¶� | ��ֹ�¶�
| �¶Ȳ�ƽ��ֵ | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55 mol��L��1NaOH��Һ��0.25 mol��L��1������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4.18 J��g��1������1�������ϱ����ݼ����к��Ȧ�H��________(ȡС�����һλ)��
���к��Ȳⶨʵ���У����в���һ���ή��ʵ��ȷ�Ե���______��
A���ζ��ܣ���������������������0.01��ȡ���������Һ�����
B��NaOH��Һ�ڵ���С�ձ�ʱ������������
C����С�ձ�������ϴв��ŵ�����ĭ���Ͻ϶�
D������������Һ���¶ȼ���ˮϴ�����������NaOH��Һ���¶�