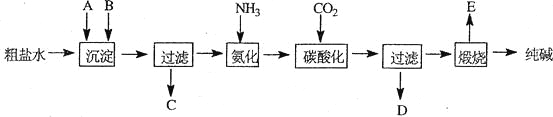
��Ŀ����
����Ŀ��ʵ��������500mL0.1mol/LNa2CO3����Һ�ش���������
��1������Na2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס���Ͳ��___��___��___��
��2��������Һ�������£�
A.��������ƽ��ȡNa2CO3��10H2O����___g��
B.�����������ձ��У�����Ͳ�����м�ˮ�ܽ⣬���ָ������£�
C.�ò���������������Һת��������ƿ��
D.ϴ�Ӳ��������ձ�2-3�Σ���ϴ��Һ��ת��������ƿ�У�
E.___��
F.����������ƿ��עˮ����Һ��������ƿ���̶�����___cmʱ������___�μ�����ˮ��Һ����̶������У�
G.�Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת���ҡ�ȣ�
H.����Һת�����Լ�ƿ�У����ϱ�ǩ����ע�Լ����ơ�Ũ�ȼ�����ʱ�䡣
��3����ʵ���������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
A.��ˮʱ�����̶���___��
B.�ܽ��δ��ȴ�����¾�ת������ƿ___��
C.����ƿ�ڱڸ���ˮ���δ���ﴦ��___��
D.����ʱ����___��
E.���µߵ�ҡ�Ⱥ�Һ����ڿ̶���___��
���𰸡�500mL����ƿ ������ ��ͷ�ι� 14.3g ����ҡ������ƿ��ʹ��Һ��Ͼ��� 1-2 ��ͷ�ι� ƫ�� ƫ�� ���� ƫ�� ����
��������
��1������500mL0.1mol/LNa2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס���Ͳ��500mL����ƿ������������ͷ�ιܣ�
��Ϊ��500mL����ƿ������������ͷ�ιܣ�
��2��������Һ�������£�
A. 500mL0.1mol/LNa2CO3��Һ������Na2CO3�����ʵ���=0.5L��0.1mol/L=0.05mol����0.05mol̼���Ƶ�Na2CO3��10H2O������=0.05mol��286g/mol=14.3g������������ƽ��ȡNa2CO3��10H2O����14.3g��
E. ����ҡ������ƿ��ʹ��Һ��Ͼ��ȣ�
F.����������ƿ��עˮ����Һ��������ƿ���̶�����1-2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������У�
��Ϊ��14.3g������ҡ������ƿ��ʹ��Һ��Ͼ��ȣ�1-2����ͷ�ιܣ�
��3��A.��ˮʱ�����̶�������Һ�������ƫ����c=![]() ����ҺŨ��ƫ�ͣ�
����ҺŨ��ƫ�ͣ�
B.�ܽ��δ��ȴ�����¾�ת������ƿ������Һ�ָ������£���Һ�������С������c=![]() ����ҺŨ��ƫ�ߣ�
����ҺŨ��ƫ�ߣ�
C.����ƿ�ڱڸ���ˮ���δ���ﴦ������Ӱ����Һ��Ũ�ȴ�С�����Ƶ���ҺŨ�Ȳ��䣻
D.����ʱ���ӣ�������Һ�����ƫ����c=![]() ����ҺŨ��ƫ�ͣ�
����ҺŨ��ƫ�ͣ�
E.���µߵ�ҡ�Ⱥ�Һ����ڿ̶��ߣ����ڿ̶������ϲ�����Һ�Σ�����һ��ʱ�䣬Һ���ָ����̶��ߣ�����Һ�ܶ���Ӱ�죬��ҺŨ�Ȳ��䣻
��Ϊ��ƫ�ͣ�ƫ�ߣ����䣻ƫ�ͣ����䡣