��Ŀ����
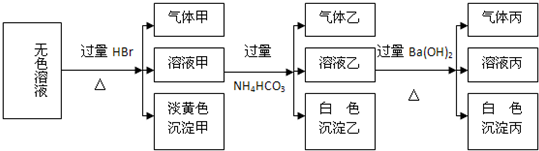
ij��ɫ��Һ�����п��ܴ���Na+��Ba2+��AlO2-��S2-��SO32-��SO42-��ȡ����Һ�����й�ʵ�飬ʵ������ͼ��ʾ��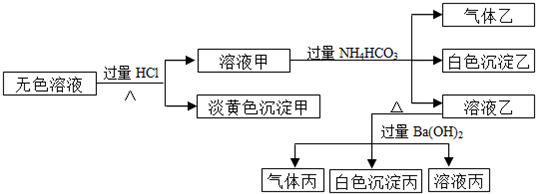
��ش��������⣺
��1������Һ�����ɳ����ҵ����ӷ���ʽΪ
��2����������һ������
��3���ۺ�������Ϣ������Һ�п϶����ڵ�������

��ش��������⣺
��1������Һ�����ɳ����ҵ����ӷ���ʽΪ
Al3++3HCO3-=Al��OH��3��+3CO2��
Al3++3HCO3-=Al��OH��3��+3CO2��
����2����������һ������
BaCO3
BaCO3
���ѧʽ����ͬ�������ܺ���BaSO4
BaSO4
����3���ۺ�������Ϣ������Һ�п϶����ڵ�������
AlO2-��S2-��SO32-��Na+
AlO2-��S2-��SO32-��Na+
����������ɫ��Һ��������������ɵ���ɫ�������ó���ֻ��ΪS��˵����Һ��S2-��SO32-�����Ի����·������з�Ӧ���ɵ���ɫ�ij���������2S2-+SO32-+6H+=3S��+3H2O����Һ����̼��������ɰ�ɫ���������壬ֻ��Al3+��HCO3-֮���ܷ���˫ˮ�ⷴӦ����Al��OH��3��ɫ��������Al3++3HCO3-=Al��OH��3��+3CO2��������Al3+��S2-�����棬����Al3+��AlO2-�����ᷴӦ��õ��ģ���Һ��һ������AlO2-������Ba2���Դ˽����⣮
����⣺��1��ֻ��Al3+��HCO3-֮���ܷ���˫ˮ�ⷴӦ����Al��OH��3��ɫ��������Al3++3HCO3-=Al��OH��3��+3CO2��������Al3+��S2-�����棬����Al3+��AlO2-�����ᷴӦ��õ��ģ���Һ��һ������AlO2-��
�ʴ�Ϊ��Al3++3HCO3-=Al��OH��3��+3CO2����
��2��������̼����狀�����������Ӧһ��������һ������̼�ᱵ�����������ʱ��Ҳ��ͱ����ӷ�Ӧ�������ᱵ���������Գ�����������BaCO3�� BaCO3��BaSO4�Ļ���
�ʴ�Ϊ��BaCO3��BaSO4��
��3������ȷ����Һ��һ������AlO2-��S2-��SO32-��������Һ�ĵ�����ԭ����һ���ú��������ӣ�����Һ������Ba2+��һ�����������ӣ��ʴ�Ϊ��AlO2-��S2-��SO32-��Na+��
�ʴ�Ϊ��Al3++3HCO3-=Al��OH��3��+3CO2����
��2��������̼����狀�����������Ӧһ��������һ������̼�ᱵ�����������ʱ��Ҳ��ͱ����ӷ�Ӧ�������ᱵ���������Գ�����������BaCO3�� BaCO3��BaSO4�Ļ���
�ʴ�Ϊ��BaCO3��BaSO4��
��3������ȷ����Һ��һ������AlO2-��S2-��SO32-��������Һ�ĵ�����ԭ����һ���ú��������ӣ�����Һ������Ba2+��һ�����������ӣ��ʴ�Ϊ��AlO2-��S2-��SO32-��Na+��
���������⿼�鳣�����ӵļ��鼰������ɷֵ�ȷ������Ŀ�Ѷ��еȣ�ע�ⳣ�����ӵļ��鷽���������й����ӵ����ʣ�����д��Ӧ�����ӵķ���ʽ��

��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
�����Ŀ