��Ŀ����
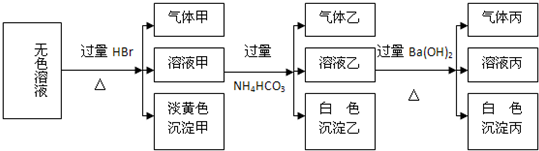
ij��ɫ��Һ�����п��ܴ����������ӣ�Na+��Ag+��Ba2+��Al3+��AlO2-��S2-��CO32-��SO32-��SO42-����ȡ����Һ�����й�ʵ�飬�����ͼ��ʾ�����ס��ҡ�������һ�����壩

�Իش��������⣺
��1�����ɵ���ɫ���������ӷ���ʽΪ
��2������Һ�����ɳ����ҵ����ӷ���ʽ
��3����������
��4������ijɷֿ��������
��5���ۺ�������Ϣ�����Կ϶����ڵ�������

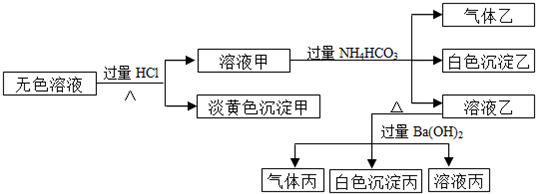
�Իش��������⣺
��1�����ɵ���ɫ���������ӷ���ʽΪ
6H++2S2-+SO32-�T3S��+3H2O
6H++2S2-+SO32-�T3S��+3H2O
����2������Һ�����ɳ����ҵ����ӷ���ʽ
Al3++3HCO3-�TAl��OH��3��+3CO2��
Al3++3HCO3-�TAl��OH��3��+3CO2��
����3����������
BaCO3����BaCO3��BaSO4��
BaCO3����BaCO3��BaSO4��
����4������ijɷֿ��������
3
3
�֣���5���ۺ�������Ϣ�����Կ϶����ڵ�������
Na+��S2-��SO32-��AlO2-
Na+��S2-��SO32-��AlO2-
��һ�������ڵ�������Ag+��Ba2+��Al3+
Ag+��Ba2+��Al3+
����������ɫ��Һ������廯�ⷴӦ����������͵���ɫ���������������廯��������S2-��CO32-��SO32-���Ͳ����������壬���Ե���ɫ����Ϊ���������������������Ӧ���ɵģ���Һ��һ�����������Ӻ�����������ӣ�
��Һ���к��й������廯�⣬�������̼����立�Ӧ�����ɵ�������Ϊ������̼����ɫ����ֻ��Ϊ������������Һ�����������������Һ��Ӧ�����ɵ������Ϊ��������ɫ����Ϊ̼�ᱵ�������ᱵ��
��1������ɫ����Ϊ���������������������ӡ�����������ӷ�Ӧ���ɵģ�
��2����Һ���к��������ӣ��������ܹ���̼��������ӷ���˫ˮ����������������
��3���������ܹ���̼������Ӻ��������������̼�ᱵ�����ᱵ������
��4�������ܹ����廯����������������������ӡ�����������Ӻ�̼������ӣ�
��5�����������ƶ��ж�һ�����ں�һ�������ڵ����ӣ�
��Һ���к��й������廯�⣬�������̼����立�Ӧ�����ɵ�������Ϊ������̼����ɫ����ֻ��Ϊ������������Һ�����������������Һ��Ӧ�����ɵ������Ϊ��������ɫ����Ϊ̼�ᱵ�������ᱵ��
��1������ɫ����Ϊ���������������������ӡ�����������ӷ�Ӧ���ɵģ�
��2����Һ���к��������ӣ��������ܹ���̼��������ӷ���˫ˮ����������������
��3���������ܹ���̼������Ӻ��������������̼�ᱵ�����ᱵ������
��4�������ܹ����廯����������������������ӡ�����������Ӻ�̼������ӣ�
��5�����������ƶ��ж�һ�����ں�һ�������ڵ����ӣ�
����⣺������ɫ��Һ������廯�ⷴӦ����������͵���ɫ���������������廯��������S2-��CO32-��SO32-���Ͳ�������������ף����Ե���ɫ����Ϊ���������������������Ӧ���ɵģ���Һ��һ�����������Ӻ�����������ӣ�һ���������Ag+��Ba2+��Al3+��
��Һ���к��й������廯�⣬�������̼����立�Ӧ�����ɵ�������Ϊ������̼����ɫ����ֻ��Ϊ����������˵��ԭ��Һ��һ������ƫ��������ӣ�
��Һ�����������������Һ��Ӧ�����ɵ������Ϊ��������ɫ����Ϊ̼�ᱵ�����ᱵ��
������Һ��һ�����ڵ������У�Na+��S2-��SO32-��AlO2-�� һ�������ڵ�����Ϊ��Ag+��Ba2+��Al3+�����ܴ��ڵ�����Ϊ��CO32-��SO42-��
��1�����ɵĵ���ɫ����Ϊ������Ӧ�����ӷ���ʽΪ��6H++2S2-+SO32-�T3S��+3H2O��
�ʴ�Ϊ��6H++2S2-+SO32-�T3S��+3H2O��
��2�������ƶϿ�֪����Һ���к��������ӣ��ܹ���̼������е�̼��������ӷ���˫ˮ�ⷴӦ��������������������Ӧ�����ӷ���ʽΪ��Al3++3HCO3-�TAl��OH��3��+3CO2����
�ʴ�Ϊ��Al3++3HCO3-�TAl��OH��3��+3CO2����
��3����ɫ��������һ�����й�����̼�����������������Ӧ���ɵ�̼�ᱵ���������ܺ���ԭ��Һ�е�����������뱵�������ɵ����ᱵ��
�ʴ�Ϊ��BaCO3����BaCO3��BaSO4����
��4�������廯�����������������ɵ������Ϊ���⣻������������ӹ��������ɵ������Ϊ�������������ɵĶ����������Ȼ���ǡ�÷�Ӧ�������ɵ������Ϊ������̼�����Կ��������3�֣�
�ʴ�Ϊ��3��
��5���������Ϸ�����ԭ��Һ��һ�����ڵ������У�Na+��S2-��SO32-��AlO2-�� һ�������ڵ�����Ϊ��Ag+��Ba2+��Al3+��
�ʴ�Ϊ��Na+��S2-��SO32-��AlO2-�� Ag+��Ba2+��Al3+��
��Һ���к��й������廯�⣬�������̼����立�Ӧ�����ɵ�������Ϊ������̼����ɫ����ֻ��Ϊ����������˵��ԭ��Һ��һ������ƫ��������ӣ�
��Һ�����������������Һ��Ӧ�����ɵ������Ϊ��������ɫ����Ϊ̼�ᱵ�����ᱵ��
������Һ��һ�����ڵ������У�Na+��S2-��SO32-��AlO2-�� һ�������ڵ�����Ϊ��Ag+��Ba2+��Al3+�����ܴ��ڵ�����Ϊ��CO32-��SO42-��
��1�����ɵĵ���ɫ����Ϊ������Ӧ�����ӷ���ʽΪ��6H++2S2-+SO32-�T3S��+3H2O��
�ʴ�Ϊ��6H++2S2-+SO32-�T3S��+3H2O��
��2�������ƶϿ�֪����Һ���к��������ӣ��ܹ���̼������е�̼��������ӷ���˫ˮ�ⷴӦ��������������������Ӧ�����ӷ���ʽΪ��Al3++3HCO3-�TAl��OH��3��+3CO2����
�ʴ�Ϊ��Al3++3HCO3-�TAl��OH��3��+3CO2����
��3����ɫ��������һ�����й�����̼�����������������Ӧ���ɵ�̼�ᱵ���������ܺ���ԭ��Һ�е�����������뱵�������ɵ����ᱵ��
�ʴ�Ϊ��BaCO3����BaCO3��BaSO4����
��4�������廯�����������������ɵ������Ϊ���⣻������������ӹ��������ɵ������Ϊ�������������ɵĶ����������Ȼ���ǡ�÷�Ӧ�������ɵ������Ϊ������̼�����Կ��������3�֣�
�ʴ�Ϊ��3��
��5���������Ϸ�����ԭ��Һ��һ�����ڵ������У�Na+��S2-��SO32-��AlO2-�� һ�������ڵ�����Ϊ��Ag+��Ba2+��Al3+��
�ʴ�Ϊ��Na+��S2-��SO32-��AlO2-�� Ag+��Ba2+��Al3+��
���������⿼���˳��������ӷ�Ӧ�����ӷ���ʽ����д������ؼ��Ǻ�������������Ϣ��������ѧ֪ʶ�õ���ȷ���ۣ�ע���ƶϵ������ԣ������Ѷ��еȣ�

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ