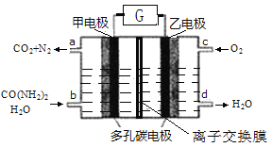
��Ŀ����
����Ŀ��H��һ�ְ����ᣬ��ϳ�·�����£�

��֪��
��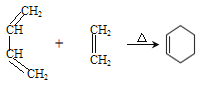
��RMgBr RCH2CH2OH+
RCH2CH2OH+![]()
��R-CHO![]()
![]()
![]()
![]()
���������գ�
��1��A�ķ���ʽΪC3H4O����ṹ��ʽΪ____________��
��2��E��F�Ļ�ѧ����ʽΪ____________��
��3��H�Ľṹ��ʽΪ_________________��д���������������ı�������ͬ���칹��Ľṹ��______________��________________��
I.���б�����II.�����������ֲ�ͬ��������ԭ�ӡ�
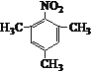
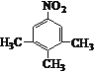
��4��������������Ϣ�����һ����CH2Cl2�ͻ������飨![]() ���Ʊ�1��4-���ϩ�ĺϳ�·�ߣ����Լ���ѡ����___________�����ϳ�·�߳��õı�ʾ��ʽΪ��A
���Ʊ�1��4-���ϩ�ĺϳ�·�ߣ����Լ���ѡ����___________�����ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B����
B����![]() Ŀ����
Ŀ����
���𰸡�CH2=CHCHO 2![]() +O2
+O2![]() 2
2![]() +2H2O
+2H2O ![]()
 CH2Cl2
CH2Cl2 ![]() ClMgCH2MgCl
ClMgCH2MgCl ![]() HOCH2CH2CH2CH2CH2OH
HOCH2CH2CH2CH2CH2OH![]() H2C=CHCH2CH=CH2
H2C=CHCH2CH=CH2
��������
A�ķ���ʽΪ![]() ����1��
����1��![]() ����ϩ��A��Ӧ����B����B��������Ӧ�õ�
����ϩ��A��Ӧ����B����B��������Ӧ�õ�![]() �������Ϣ
�������Ϣ![]() ��֪AΪ
��֪AΪ![]() ��BΪ
��BΪ![]()
![]() ���ת����ϵ����Ϣ
���ת����ϵ����Ϣ![]() ��֪
��֪![]() ����ȡ����Ӧ����CΪ
����ȡ����Ӧ����CΪ![]() ��DΪ
��DΪ![]() ��EΪ
��EΪ![]() ������Ϣ
������Ϣ![]() ��֪FΪ
��֪FΪ![]() ��GΪ
��GΪ![]() ��HΪ
��HΪ![]() ��
��
��1��A�ķ���ʽΪ![]() ����ṹ��ʽΪ��
����ṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2��E��F�ǻ��Ĵ���������Ӧ�Ļ�ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3��H�Ľṹ��ʽΪ![]() ���������������ı�������ͬ���칹��Ľṹ��ʽ�����б����������������ֲ�ͬ��������ԭ�ӣ�����������ͬ���칹���У�
���������������ı�������ͬ���칹��Ľṹ��ʽ�����б����������������ֲ�ͬ��������ԭ�ӣ�����������ͬ���칹���У� ��
�� �� �ʴ�Ϊ��
�� �ʴ�Ϊ��![]() ��
�� ��
�� ��
��
��4����![]() ��
��![]() ���ѵõ�
���ѵõ�![]() �����뻷������õ���
�����뻷������õ���![]() ��Ȼ����Ũ������������·�����ȥ��Ӧ�õ�
��Ȼ����Ũ������������·�����ȥ��Ӧ�õ�![]() ���ϳ�·������ͼΪ��
���ϳ�·������ͼΪ��![]() �� �ʴ�Ϊ��
�� �ʴ�Ϊ��![]() ��
��

����Ŀ����������ĵ��볣�����±���
���� | HCOOH | HClO | H2CO3 | H2SO3 |
���볣�� ��25�棩 |
|
|
|
|
��1��ͬ��ͬ���ʵ���Ũ�ȵ�HCOONa��aq����NaClO��aq����pH�����________��
��2��1molCl2��2molNa2CO3��aq����Ӧ������NaCl���_______________���ѧʽ����
��3����һ������NaHCO3��aq����ͨ��������SO2��g������Ӧ�����ӷ���ʽΪ__________�������ᣨH2SeO3��Ҳ��һ�ֶ�Ԫ���ᣬ��������һ����ɫ���壬������ˮ���н�ǿ�������ԡ�
����Ŀ�������̿��Ʊ�������ص���Ҫ��Ӧ���£�
�������� 3MnO2+KClO3+6KOH![]() 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O
�����绯 3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3
��֪������ʵ��ܽ�ȣ�20����
���� | K2CO3 | KHCO3 | K2SO4 | KMnO4 |
�ܽ��g/100g | 111 | 33.7 | 11.1 | 6.34 |
���������գ�
��1����ʵ���ҽ�������������������ʱ��Ӧѡ������������ǯ��______________��������ţ�
a�������� b�������� c�������� d��������
��2����������᪻���ʱ�����������ԭ����__________�������������ԭ����____________����Ӧ֮��õ�������صIJ����ǣ����ˡ������ᾧ�����ȹ��ˡ��ò����ܹ��õ�������ص�ԭ����____________��
��3�����õ�ⷨҲ��ʵ��K2MnO4��ת����2K2MnO4+2H2O![]() 2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ_________________��
2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ_________________��
��4�������Ƶζ����ⶨ������ص����������������£�
��֪�漰���ķ�Ӧ��
Na2C2O4+H2SO4=H2C2O4(����)+Na2SO4 ��
5H2C2O4+ 2MnO4��+6H+=2Mn2++10CO2��+ 8H2O
Na2C2O4��ʽ����134 KMnO4��ʽ����158��
��.��ȡ0.80 g �ĸ�����ز�Ʒ�����50mL��Һ��
��.��ȡ0.2014 gNa2C2O4��������ƿ�У���������ˮʹ���ܽ⣬�ټ������������ữ��
��.��ƿ����Һ���ȵ�75��80�����������������Ƶĸ��������Һ�ζ����յ㡣���ĸ��������Һ8.48mL��
a.�жϴﵽ�ζ��յ�ı�־��____________________________��
b.��Ʒ�и�����ص���������Ϊ______________������3λС������
c.�����¶ȴ���90�������ֲ��ᷢ���ֽ⣬�ᵼ�²�ò�Ʒ����__________��������ƫ��������ƫ����������Ӱ������
d.��һ�������������Һ���ữ�IJ�������Һ��ϣ���÷�Ӧ��Һ��Mn2+��Ũ���淴Ӧʱ��t�ı仯��ͼ����ԭ�����Ϊ_______________��

����Ŀ������������ⶪ�����������ˮ������Ⱦ������к��е��ؽ������Ӷ�ֲ�������ж������ã��������廹���˺��˵���ϸ�������Ρ����������ȵȡ�ijͬѧ����һЩ�����ﳣ���µ��ܶȻ��������±���
���� | FeS | CuS(��ɫ) | MnS(��ɫ) | PbS | HgS | ZnS |
Ksp |
|
|
|
|
|
|
���� | Fe(OH)3 | Al(OH)3 | Mn(OH)2 | Pb(OH)2 | Zn(OH)2 | |
Ksp |
|
|
|
|
|
(1)��ͬѧ�ں�����ͬŨ��Mn2+��Cu2+����Һ�еμ�Na2S��ϡ��Һ���۲쵽�ȳ��ֵij�����ɫ��______����ʵ��˵������ͬ������KspԽС�����ʵ��ܽ��____(����Խ��������ԽС��)��
(2)��֪�����������ӿ�ʼ������pHΪ1.0�������Һ�� Fe3+�����ʵ���Ũ��Ϊ______������Һ�к�����Fe3+��Ũ�ȵ� Al3+������ pH ʹ Fe3+������ȫʱ������_____Al(OH)3����(����������������������)��
(3)ij��ҵ��ˮ�к���Cu2+��Pb2+��Hg2+���ʣ����ó���ת��ԭ�������˼��������__________(�����)��д��������Լ���ȥPb2+ʱ���������ӷ�Ӧ����ʽ_______��
A.FeS B.Fe2O3 C.Na2S D.ZnO
(4)�� FeCl3��6H2O����õ�������ˮFeCl3����Ҫ���еIJ�����_________��