��Ŀ����
����Ŀ��������ҵ�г��ô���ͭ����Һ{��[Cu(NH3)2]+��CH3COO����NH3}��ȥH2�е�CO��O2��H2S���塣
��1����֪����ͭ����Һ����CO�ķ�ӦΪ��
[Cu(NH3)2]+(aq)+CO(g)+NH3(g) ![]() [Cu(NH3)3��CO]+(aq)
[Cu(NH3)3��CO]+(aq)
����Ӧ�ں����ܱ������н��У�����˵���÷�Ӧ�Ѵﵽƽ��״̬���� ��
a��v(CO)��=v(NH3)��
b������������ѹǿ������ʱ��仯
c��[Cu(NH3)2]+Ũ�Ȳ�����ʱ��仯
��2������ͭ����Һ����COһ��ʱ���ʧЧ�������з����ɻ��շϴ���ͭ����Һ�е�ͭ��
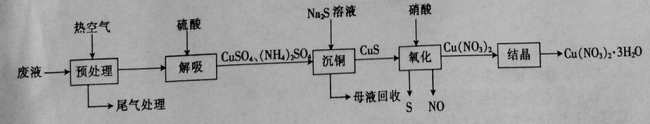
��Ԥ������ʱ��β���г�N2��CO��O2��CO2��H2O�⣬���� ��
������������Һʱ������Ӧ�����ӷ���ʽΪ ��
����������ʱ������Ӧ�Ļ�ѧ����ʽΪ ��
����֪����������ֻ������ͭ�������ᾧ������ͭԪ������ġ�����ͭ��ʱͭԪ�ص������Ϊ4%�����ᾧ��ʱͭԪ�ص������Ϊ2%����1L��Һ�����Ƶ�Cu(NO3)2��3H2O 363g����1L��Һ��ͭԪ�ص�����Ϊ g��
���𰸡���1��bc
��2����NH3 ��Cu2++S2��=CuS��
��3CuS+8HNO3=3Cu(NO3)2+2NO��+3S��+4H2O ��102
��������
�����������1������Ӧ�ں����ܱ������н��У�����˵���÷�Ӧ�Ѵﵽƽ��״̬���ǣ�a��v(CO)��=v(NH3)������һ��v��=v������һ��ƽ�⣻
b����Ӧ![]() ��ѹǿ�DZ���������������ѹǿ������ʱ��仯��һ��ƽ�⣻
��ѹǿ�DZ���������������ѹǿ������ʱ��仯��һ��ƽ�⣻
c��[Cu(NH3)2]+Ũ�Ȳ�����ʱ��仯��һ��ƽ�⣻
��2������Һ����[Cu(NH3)3��CO]+����Ԥ������ʱ��β���г�N2��CO��O2��CO2��H2O�⣬���а�����������������Һ���ɺ�ɫ��ͭ������������Ӧ�����ӷ���ʽΪ��Cu2++S2��=CuS����������ͼʾ����������ʱ����ͭ������Ϊ����ͭ�����ʣ�������Ӧ�Ļ�ѧ����ʽΪ3CuS+8HNO3=3Cu(NO3)2+2NO��+3S��+4H2O��������Ԫ���غ㣬1L��Һ��ͭԪ�ص�����Ϊ363g��242g/mol��96%��98%��64g/mol=102g��

����Ŀ��һ���¶��£�2L�ܱ������г���0.40 mol N2O4��������Ӧ��N2O4��g��![]() 2NO2��g����һ��ʱ���ﵽƽ�⣬����������£�
2NO2��g����һ��ʱ���ﵽƽ�⣬����������£�
ʱ�䣯s | 20 | 40 | 60 | 80 | 100 |
c��NO2 ��/��mol/L�� | 0��12 | 0��20 | 0��26 | 0��30 | 0.30 |
��ش�
��1��20 s�ڣ�v��NO2��=____________mol��L-1��s-1��
��2�������¶�ʱ��������ɫ���������Ӧ��____________������ȡ������ȡ�����Ӧ��
��3�����¶��·�Ӧ�Ļ�ѧƽ�ⳣ����ֵΪ_____________��
��4����ͬ�¶��£�����ʼʱ��������г������0.20 molN2O4��0.40molNO2����ﵽƽ���c��NO2��=_____________��
����Ŀ������ʵ�����������ͽ��Ͷ���ȷ���ǣ� ��
ѡ�� | ʵ����� | ���� | ���� |
A | ��ij��Һ�м������� | ������ɫ���� | ��Һ��һ������CO32 |
B | ���ѻ������м������Ը��������Һ���� | ��ɫ��ȥ | ˵�������к��мױ��ȱ���ͬϵ�� |
C | �ô�����Һ����ˮ�� | ˮ���ܽ⣬����ɫ���� | ���ԣ�����>̼�� |
D | ���м���Ũ��ˮ�������Ʊ��屽 | ��Һ�����ڣ��²������״Һ�� | �����±���Ũ��ˮ������ȡ����Ӧ |
