��Ŀ����
16��������ѧ��ѧ���֪ʶ�������ƶ���ȷ��һ���ǣ�������| A�� | ��A��Ԫ���γɵ��⻯��ķе���ϵ������ε��������A��Ԫ�����γɵ��⻯��ķе���ϵ���Ҳ�����ε��� | |
| B�� | �������û���KBr��Һ�е��壬�����Ҳ���û���KBr��Һ�е��� | |
| C�� | ijŨ�ȵ�NaClO��Һ��pH=d����������ˮ�������c��H+��=10-14+d mol•L-1 | |
| D�� | ij�¶��£�MgCO3��Ksp=6.8��10-6������¶������к�����MgCO3����Һ������c��Mg2+��=c��CO32-������c��Mg2+��•c��CO32-��=6.8��10-6 |
���� A��������γ�ʹ�۷е����ߣ�
B����������ˮ��Ӧ����������
C��NaClO��Һˮ���Լ��ԣ�����Һ�е�����������Ϊˮ����ģ���ˮ�������c��H+��=c��OH-�������˽��м��㣻
D��������þ���ӵ���Һ�У�c��Mg2+����c��CO32-����
��� �⣺A����A��Ԫ���γɵ��⻯��ķе���ϵ������ε���������A��Ԫ�����γɵ��⻯���У�����NH3���Ӽ���γ�������ʷе���������⻯���A����
B���������û���KEr��Һ�е��壬����������ˮ��Ӧ�����������ʷ��������û���KBr�е��壬��B����
C��ijŨ�ȵ�NaClO��Һ��pH=d������Һ��������Ũ��Ϊ��c��H+��=1��10-dmol/L����c��OH-��=10-14+dmon•L-1����Һ�е�����������Ϊˮ����ģ�������ˮ�����������Ũ��Ϊc��OH-��=10-14+dmon•L-1����C��ȷ��
D������Һ��þ����Ũ�Ⱥܴ�������ܽ�ƽ�������ƶ���c��Mg2+����c��CO32-�������¶Ȳ��䣬c��Mg2+��•c��CO32-��=6.8��10-6����D����
��ѡC��
���� ���⿼����������ʡ�±�嵥�ʵ����ʡ������ˮ�⣬���������ܽ�ƽ���֪ʶ���ѶȲ���Ҫע������������������ʵ�Ӱ�죮

| A�� | ���Ӱ뾶��X+��Y- | |
| B�� | ZԪ�ص���������ϼ���������ϼ۴�����Ϊ4 | |
| C�� | X��Y��Z�����γ�XYZ��XYZ3��XYZ4�Ȼ����� | |
| D�� | Y���⻯���д������Ӽ� |
| A�� | Na3N�����ᷴӦʱֻ����һ����NaCl | |
| B�� | ��Na3N��ˮ��Ӧ��Na3N�ǻ�ԭ�� | |
| C�� | Na3N������Na+�İ뾶��N3-�İ뾶С | |
| D�� | Na+��N3-�ĵ��Ӳ㶼���ԭ�ӵĽṹ��ͬ |
| A�� | ��ˮ�м��������������ƹ��壬�ٽ���ˮ�ĵ��룬c��H+������Kw���� | |
| B�� | pH=8��NaHCO3��Һ�У�c��OH-��=1��10-6mol/L | |
| C�� | ������ˮ�У�c��Cl-��=c��ClO-��+c��HClO�� | |
| D�� | 0.1mol/L�ģ�NH4��2SO4��Һ�У�c��SO42-����c��NH4+����c��H+����c��OH-�� |
| A�� | һ������Na2O��Na2O2��NaHCO3 | B�� | һ������Na2O��NaCl | ||
| C�� | ������Na2CO3��NaCl | D�� | ������NaHCO3��Na2CO3 |
| A�� | ϴ�ˡ�ϴ�¡�����ˮ�����������ϵء������ | |
| B�� | ��ǿ��ҵ��ˮ���ŷż�أ���ִ���ŷ� | |
| C�� | ����ʩ��ũҩ�����ʣ��Լ���ˮ����Ⱦ | |
| D�� | ��ˮ��Դ�ḻ�����Ե�ˮ����ȡ֮��������֮���� |
| A�� | ���Ƿǽ���Ԫ�أ�������ǻҺ�ɫ�н�������Ĺ��� | |
| B�� | ��ĵ������ܽ��ڽ����;�Ե��֮�䣬�����õİ뵼����� | |
| C�� | ��Ļ�ѧ���ʲ����ã������²����κ����ʷ�Ӧ | |
| D�� | �������賣������������ά |
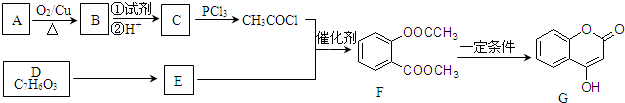
 ��
��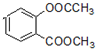 +3NaOH��CH3COONa+CH3OH+
+3NaOH��CH3COONa+CH3OH+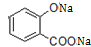 +H2O��
+H2O��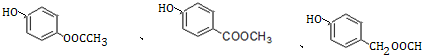 ��
��