��Ŀ����
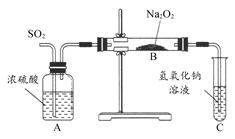
������ʵ��С���ͬѧΪ̽�������������������ķ�Ӧ��������ͼ��ʾ��װ�ý���ʵ�顣ͨ��SO2���壬�������ǵ�ľ�������Թ�C�е���Һ�Ϸ���ľ����ȼ��
��ش��������⣺
(1)��1С��ͬѧ��ΪNa2O2��SO2��Ӧ������Na2SO3��O2���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(2)�����һ��ʵ�鷽��֤��Na2O2��SO2��Ӧ���ɵİ�ɫ�����к���Na2SO3�� ��
(3)��2С��ͬѧ��ΪNa2O2��SO2��Ӧ��������Na2SO3��O2�⣬����Na2SO4���ɡ�Ϊ�����Ƿ���Na2SO4���ɣ�������������·�����
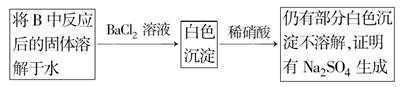
���������Ƿ������ �����Ҫ˵���������ɣ��� ���� ��

��ش��������⣺
(1)��1С��ͬѧ��ΪNa2O2��SO2��Ӧ������Na2SO3��O2���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(2)�����һ��ʵ�鷽��֤��Na2O2��SO2��Ӧ���ɵİ�ɫ�����к���Na2SO3�� ��
(3)��2С��ͬѧ��ΪNa2O2��SO2��Ӧ��������Na2SO3��O2�⣬����Na2SO4���ɡ�Ϊ�����Ƿ���Na2SO4���ɣ�������������·�����

���������Ƿ������ �����Ҫ˵���������ɣ��� ���� ��
(1)2Na2O2��2SO2=2Na2SO3��O2 (2)ȡ��ɫ��������������ϡ���ᣬ��������ʹƷ����Һ��ɫ�����壬��֤���ù����к���Na2SO3 (3)������ ��ϡ�����ܽ������ᱵ���������ᱵ �������Ӧ��Ĺ����в�����Na2O2��������ˮ��Ҳ�ܽ�������������������
������Na2O2��SO2��Ӧ��ԭ��Ϊ����������֪ʶ��Ǩ��������(1)���CO2��Na2O2��Ӧ�Ļ�ѧ����ʽ������д��SO2��Na2O2��Ӧ�Ļ�ѧ����ʽΪ��2Na2O2��2SO2=2Na2SO3��O2��(2)����SO32-��������ɫ�����м���ϡH2SO4�����ܷ����ʹƷ����Һ��ɫ����ɫ�д̼�����ζ�����壬�������������壬��֤����Na2SO3���ɡ�(3)HNO3(ϡ)����ǿ�����ԣ��ɽ�BaSO3������BaSO4������Ӧ��Ĺ���������Na2O2��������ˮ��Ҳ�ܽ�SO32-������SO42-��������������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
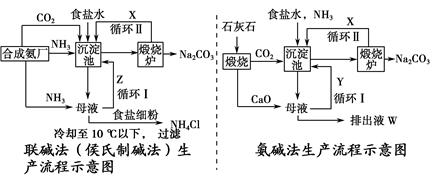
 Na2CO3��H2O����CO2��
Na2CO3��H2O����CO2��