��Ŀ����
����Ŀ����������(NaClO2)��һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������£�
��1�����з�����Ӧ�Ļ�ԭ����____ (�ѧʽ)��
��2�����з�Ӧ�����ӷ���ʽ��____��
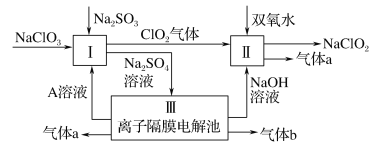
��3���������Ӹ�Ĥ���ص�װ�����£�
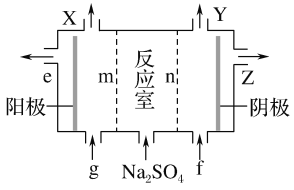
��A�Ļ�ѧʽ��________��A��____�ڲ�����
��mΪ____ (����������������)���ӽ���Ĥ��
�۽�ϻ�ѧ���������˵������NaOH������ԭ��________
��4��ClO2��һ�ָ�Чˮ�������������������ƺ�ϡ����Ϊԭ���Ʊ���5NaClO2��4HCl=5NaCl��4ClO2����2H2O
�ٸ÷�Ӧ���������ͻ�ԭ�������ʵ���֮����____��
���о�����������Ӧ��ʼʱ����Ũ��Խ�������������Cl2�ĺ���Խ������������ԭ��Ӧ���ɷ�����ԭ����____��
��5��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2�������ʵ���ǰ��____ (����>����<����������)���ߡ�
���𰸡�Na2SO3 2ClO2��H2O2��2OH��=2ClO2-��O2����2H2O H2SO4 e �� ������ӦΪ2H2O��2e��=H2����2OH������Ӧ���е�Na���������ӽ���Ĥ���������ң���������������OH������NaOH 1��4 ClO2-�������Ի�Cl���Ļ�ԭ������Һ�����Ժ�Ũ�ȵ��������ǿ�����Cl����������Cl2 ��
��������
��������Һͨ�����Ӹ�Ĥ�����У�ClO2��˫��ˮ��II�з���������ԭ��Ӧ����NaClO2������a���÷�Ӧ��ClԪ�ػ��ϼ���+4�۱�Ϊ+3�ۣ���OԪ�ػ��ϼ���-1�۱�Ϊ0�ۣ��������ɵ�����a��O2�����ӷ�Ӧ����ʽΪ2ClO2+H2O2+2OH-=2 ClO2-+O2��+2H2O��III�з�����⣬����ͼ֪�������������ƣ���������������ͬʱ������������NaOH���������ɵ�����b��H2�����������������ӷŵ���������������a��O2��ͬʱ�������ᣬ����A��Һ��������������£�I��NaClO2��Na2SO3����������ԭ��Ӧ�����ӷ�Ӧ����ʽΪ2ClO3-+2H++SO32-=2ClO2��+SO42-+H2O��
(1)��ͼ�õ����н���������ת��Ϊ�����ƣ�S�Ļ��ϼ��ɣ�4����Ϊ��6�����Ի�ԭ��Ϊ�������ƣ�
(2)���н����ClO2ת��ΪNaClO2���ϼ۽��ͣ�������H2O2�еģ�1�۵������ϼ�����Ϊ0��O2������ʽΪ��2ClO2��H2O2��2OH��=2ClO2-��O2����2H2O��
(3)�������Ӹ�Ĥ���أ��м������������Һ����������������������������ƶ�������m�������ӽ���Ĥ�����������ķ�ӦΪ2H2O��4e��=O2����4H����������Ũ�����ӣ���ϴ���m����������ӵõ����ᣬ��XΪ������eΪ���ᣬ�����gΪϡ����(��Ҫ��Ϊ��ʹ��Һ����)����Ӧ��nΪ�����ӽ���Ĥ���Ա�֤���������������ƶ���������ӦΪ2H2O��2e��=H2����2OH�������ɵ���������������ӵõ��������ƣ�����YΪ������ZΪ����������Һ�������fΪϡ����������Һ(��ҪΪ��ʹ��Һ����)�����������Ϣ�õ���
��A�Ļ�ѧʽ��H2SO4��A��e�ڲ�����
��mΪ�����ӽ���Ĥ��
��NaOH������ԭ��������ӦΪ2H2O��2e��=H2����2OH�������ɵ���������������ӵõ��������ƣ�
(4)�ٸ�����ͬԪ�ص���ͬ��̬һ��ֱ��ת����ԭ�����������е�NaClһ�����Է�Ӧ������ᣬNaCl��5����HClֻ��4����������1��NaCl����NaClO2������4��NaClO2ת��ΪClO2������������Ϊ1��NaClO2����ԭ��Ϊ4��NaClO2�����Ը÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ1��4��
������Ӧ��ʼʱ����Ũ��Խ�������������Cl2�ĺ���Խ��˵��ʹ��Ũ����ʱ���ᱻNaClO2����ΪCl2������������Ũ������NaClO2����������ǿ����������Ļ�ԭ����ǿ��
(5)����NaClO2�Ƿ���ʣ���������������ӵ�ʱ���ǽ�������������Ϊ�����ӣ�Cl���ն���ת��ΪCl�������ʵķ�ӦΪ3NaClO2=2NaClO3��NaCl����3molNaClO2ת��Ϊ2 mol NaClO3����2 mol NaClO3�õ���Ϊ2��6e����12 mol���ӡ���������ʣ�3 mol NaClO2�õ���Ϊ��3��4e����12 mol���ӣ�����NaClO2����ǰ���ܵõ��ĵ���ʵ����һ���ģ���������������������ͬ�ġ�