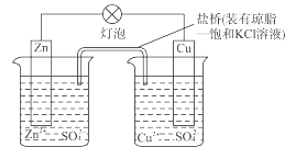
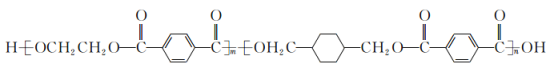��Ŀ����
����Ŀ��W��X��Y��ZΪԪ�����ڱ���ǰ������Ԫ�أ���ԭ��������������W�Ļ�̬ԭ����ռ��������ԭ�ӹ���ĵ�����Ϊ3��![]() ��
��![]() ������ͬ�ĵ��Ӳ�ṹ��W��X������������֮�͵���Y��������������ZԪ��λ��Ԫ�����ڱ��ĵ�11�У���ش�
������ͬ�ĵ��Ӳ�ṹ��W��X������������֮�͵���Y��������������ZԪ��λ��Ԫ�����ڱ��ĵ�11�У���ش�
��1��Z�Ļ�̬ԭ��M��ĵ����Ų�ʽΪ____________________��
��2��W�ļ��⻯�������Wԭ�ӵ�__________�������ԭ�ӵ�__________����ص��γ�![]() �������������ƣ�
�������������ƣ�
��3���Ƚ�Y�ĺ���������ԣ�![]() __________
__________![]() ���>����<������ԭ��Ϊ___________________��
���>����<������ԭ��Ϊ___________________��
��4������Z�Ļ�����![]() ��EDTA�Ľṹ��ʽ����ͼ����˵����ȷ����_____ѡ����ĸ����
��EDTA�Ľṹ��ʽ����ͼ����˵����ȷ����_____ѡ����ĸ����
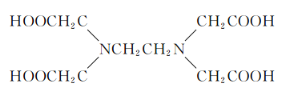
A��![]() �������Ļ�ѧ�������Ӽ������ۼ�����λ�������
�������Ļ�ѧ�������Ӽ������ۼ�����λ�������
B��EDTA��̼ԭ�ӵ��ӻ��������Ϊ![]() ��
��![]()
C��![]() �����Ԫ�صĵ�һ������˳��Ϊ
�����Ԫ�صĵ�һ������˳��Ϊ![]()
D��![]() ��
��![]() ��Ϊ�ȵ����壬�ռ乹�;�Ϊ����������
��Ϊ�ȵ����壬�ռ乹�;�Ϊ����������
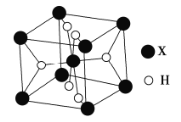
��5��ij�������⻯��![]() �ľ����ṹ��ͼ��ʾ��
�ľ����ṹ��ͼ��ʾ��
��![]() ����λ��Ϊ__________________��
����λ��Ϊ__________________��
��![]() ��һ�ִ�����ϣ���ˮ�Ỻ����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
��һ�ִ�����ϣ���ˮ�Ỻ����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
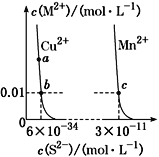
�����þ������ܶ�Ϊ![]() ���������Ϊ______________
���������Ϊ______________![]() ��
��
���𰸡�3s23p63d10 sp3 s �� HClO2��HClO�ɷֱ��ʾΪ(HO)ClO��(HO)Cl��HClO�е�ClΪ+1�ۣ���HClO2�е�ClΪ+3�ۣ������Ը��ߣ�����Cl��O��H��O�ĵ��Ӹ���Clƫ�ƣ��������H+ BD 3 MgH2+2H2O��Mg(OH)2+2H2�� ![]()
��������
W��X��Y��ZΪԪ�����ڱ���ǰ������ԭ���������������Ԫ�أ�W�Ļ�̬ԭ����ռ��������ԭ�ӹ���ĵ�����Ϊ3����WΪNԪ�أ�X2+��W3-������ͬ�ĵ��Ӳ�ṹ�����������Ӻ�����10�����ӣ���XΪMgԪ�أ�ZԪ��λ��Ԫ�����ڱ��ĵ�11�У���ZΪCuԪ�أ�Yԭ������С��29��W��X������������֮�͵���Y����������������YΪClԪ�أ��ݴ˷�����
��1��ZΪCuԪ�أ���̬ԭ��M��ĵ����Ų�ʽΪ3s23p63d10��
��2��W�ļ��⻯��ΪNH3�����������У�ԭ�Ӽ��γ�����ʱ�������ص���ԭ�ӹ���ֱ�Ϊsp3�ӻ������s�����
��3��ͬһ��Ԫ�صĺ������У����з��ǻ���ԭ�Ӹ���Խ�࣬������Խǿ��HYO2�з��ǻ�Oԭ�Ӹ�����1��HYO��û�з��ǻ���ԭ�ӣ���������HYO2��HYO���ʴ�Ϊ����HClO2��HClO�ɷֱ��ʾΪ(HO)ClO��(HO)Cl��HClO�е�ClΪ+1�ۣ���HClO2�е�ClΪ+3�ۣ������Ը��ߣ�����Cl��O��H��O�ĵ��Ӹ���Clƫ�ƣ��������H+��
��4��A��[Z(EDTA)]SO4�������Ļ�ѧ�������Ӽ������ۼ�����λ���������������ѡ��A����
B��EDTA��̼ԭ����2���ӻ�������ͣ������Ȼ���Ϊsp2���Ǽ���Ϊsp3��ѡ��B��ȷ��
C��Nԭ�ӵ�2pΪ������ṹ����һ���������ѡ��C����
D��[Z(EDTA)]SO4�������������������ӣ�S�ļ۲���Ӷ���Ϊ4+![]() (6+2-4��2)=4���ռ乹��Ϊ�������壬ѡ��D��ȷ��
(6+2-4��2)=4���ռ乹��Ϊ�������壬ѡ��D��ȷ��
��ѡBD��
��5�����������⻯��MgH2�ľ����У�ÿ�����������Ӿ����������ϵ�þ���Ӻ�һ�������ϵ�þ���ӣ���3��þ���ӣ����������ӵ���λ��Ϊ3��
��MgH2��ˮ�Ỻ����Ӧ�ų���������Ӧ�Ļ�ѧ����ʽΪ��MgH2+2H2O��Mg(OH)2+2H2����
��Mgλ�ڶ�������ģ���ĿΪ8��![]() +1=2��Hλ�����ģ���ĿΪ��8��
+1=2��Hλ�����ģ���ĿΪ��8��![]() =4��������Ϊ
=4��������Ϊ![]() g���������Ϊ��
g�����������![]() cm3=
cm3=![]() nm3��
nm3��

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�