��Ŀ����
3��X��Y��Z��W��Ԫ�����ڱ���ԭ���������������ǰ������Ԫ�أ��������Ϣ���±���| Ԫ�� | �����Ϣ |
| X | X���γɻ�������������Ԫ�أ�����һ�ֵ�������Ȼ����Ӳ������ |
| Y | Y����̬�⻯����ʹʪ��ĺ�ɫʯ����ֽ���� |
| Z | Z�Ļ�̬ԭ�ӵ����������Ų�ʽΪns2np4 |
| W | W��һ�ֺ��ص�������Ϊ57��������Ϊ31 |
��2��Z�ĵ�һ�����ܱ�Y��С�����С������XZ2��һ�ֳ��õ��ܼ���XZ2�����ЦҼ��ͦм��ĸ�����Ϊ1��1��Y����̬�⻯���Z����̬�⻯���������ˮ������Ҫԭ����NH3��ˮ���Ӽ����γ��������H2S��ˮ���Ӽ䲻���γ������
��3��WZ2�ڿ�������������W2O3�Ļ�ѧ����ʽ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��4������β���к���XO��YO����������������װ��ij�ִ�����ʹ����ת��Ϊ����Y����֪��2XO��g��+O2��g���T2XO2��g����H=-566.0kJ•mol-1��Y2��g��+O2��g���T2YO��g����H=180.0kJ•mol- 1�˷�Ӧ���Ȼ�ѧ����ʽ��2CO��g��+2NO��g���TN2��g��+2CO2��g����H=-746.0kJ•mol-1��
���� X��Y��Z��W��Ԫ�����ڱ���ԭ���������������ǰ������Ԫ�أ�X���γɻ�������������Ԫ�أ�����һ�ֵ�������Ȼ����Ӳ�����ʣ���XΪCԪ�أ�Y����̬�⻯����ʹʪ��ĺ�ɫʯ����ֽ��������YΪNԪ�أ�Z�Ļ�̬ԭ�ӵ����������Ų�ʽΪ3sn3pn+2��n=2����ZΪSԪ�أ�W��һ�ֺ��ص�������Ϊ57��������Ϊ31��������Ϊ57-31=26����WΪFe���ݴ˴��⣮
��� �⣺X��Y��Z��W��Ԫ�����ڱ���ԭ���������������ǰ������Ԫ�أ�X���γɻ�������������Ԫ�أ�����һ�ֵ�������Ȼ����Ӳ�����ʣ���XΪCԪ�أ�Y����̬�⻯����ʹʪ��ĺ�ɫʯ����ֽ��������YΪNԪ�أ�Z�Ļ�̬ԭ�ӵ����������Ų�ʽΪ3sn3pn+2��n=2����ZΪSԪ�أ�W��һ�ֺ��ص�������Ϊ57��������Ϊ31��������Ϊ57-31=26����WΪFe��
��1��WΪFeԪ�أ��������ڱ��е�������VIII�壬���̬ԭ����Χ�����Ų�Ϊ3d64s2��������4��δ�ɶԵ��ӣ��ʴ�Ϊ���ġ�VIII��4��
��2��NԪ��ԭ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���OԪ�أ���Ԫ�ص�һ�����ܸ���SԪ�أ��ʵ�һ������S��N��CS2��һ�ֳ��õ��ܼ����ṹʽΪS=C=S�������ЦҼ��ͦм��ĸ�����Ϊ1��1��NH3��ˮ���Ӽ����γ��������H2S��ˮ���Ӽ䲻���γ��������NH3��������ˮ��
�ʴ�Ϊ��С��1��1��NH3��ˮ���Ӽ����γ��������H2S��ˮ���Ӽ䲻���γ������
��3��FeS2�ڿ�������������Fe2O3�Ļ�ѧ����ʽ�ǣ�4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
�ʴ�Ϊ��4FeS2+11O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+8SO2��
��4����֪����2CO��g��+O2��g���T2CO2��g����H=-566.0kJ•mol-1��
��N2��g��+O2��g���T2NO��g����H=180.0kJ•mol-1
���ݸ�˹���ɣ���-�ڿɵã�2CO��g��+2NO��g���TN2��g��+2CO2��g����H=-746.0kJ•mol-1��
�ʴ�Ϊ��2CO��g��+2NO��g���TN2��g��+2CO2��g����H=-746.0kJ•mol-1��
���� �����ǿ���ṹ����λ�ù�ϵӦ�ã��漰��������Ų��������ܡ��������ѧ�����Ȼ�ѧ����ʽ��д�ȣ��ѶȲ���ע��Ի���֪ʶ���������գ�

| A�� | C6H6 | B�� | C7H8 | C�� | C4H10 | D�� | C3H6 |
| A�� | ��H2O | B�� | ���뱥��ʳ��ˮ | ||
| C�� | ����ѹǿ | D�� | �����¶ȣ�����������ӷ��� |
| A�� | ȷ��ȡ0.4000g��NaOH�������1000mLŨ��Ϊ0.01000 mol•L-1����Һ | |
| B�� | ����۸��ǵ������еĹ������ȼ���Һϴȥ�Թ��ڱڵ����ö�����̼��������������ʧ����ʪ��ĺ�ɫʯ����ֽ���鰱���Ƿ��������ϲ��������� | |
| C�� | �ü���ʼ���Fe��OH��3�����Fe��SCN��3��Һ | |
| D�� | SO2����ˮ����ˮ��Һ�ܵ��磬˵��SO2�ǵ���� |
| A�� | Mg | B�� | $\frac{1}{M}$g | C�� | $\frac{M}{6.02��1{0}^{23}}$g | D�� | $\frac{6.02��1{0}^{23}}{\;}$g |
| A�� | C2H4��C2H2 | B�� | CH4��C2H6 | C�� | CH4��C2H4 | D�� | C2H2��C3H6 |
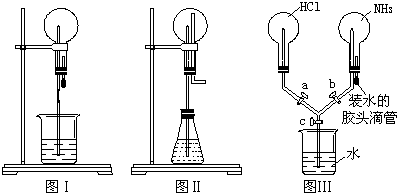

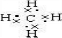 ���ռ乹�����������壬����Ľṹ��ʽΪ��CH3CH2CH3�춡��Ľṹ��ʽCH3CH��CH3��CH3
���ռ乹�����������壬����Ľṹ��ʽΪ��CH3CH2CH3�춡��Ľṹ��ʽCH3CH��CH3��CH3
 ��M�Ļ�ѧʽΪFeS��F�ĵ���ʽΪ
��M�Ļ�ѧʽΪFeS��F�ĵ���ʽΪ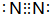 ��
��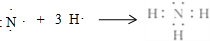 ��
��