��Ŀ����
20��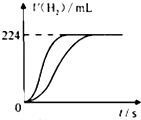 ��ȡ8.0mL 5.0mol•L-1H2SO4��Һ��������ˮϡ����100mL��ȡ����ϡ�ͺ��H2SO4��Һ��25mL���ֱ�����������Zn��Fe����ͬ�����³�ַ�Ӧ�����������������ʱ��仯��������ͼ��ʾ���������������ɱ�״���µ������������㣺
��ȡ8.0mL 5.0mol•L-1H2SO4��Һ��������ˮϡ����100mL��ȡ����ϡ�ͺ��H2SO4��Һ��25mL���ֱ�����������Zn��Fe����ͬ�����³�ַ�Ӧ�����������������ʱ��仯��������ͼ��ʾ���������������ɱ�״���µ������������㣺��1��ϡ�ͺ�H2SO4��Һ�����ʵ���Ũ��Ϊ0.40mol•L-1��
��2������Fe������������0.65g��
���� ��1��H2SO4��Һ��25mL���ֱ�����������Zn��Fe�����ɵ������������ͬ������Fe��Ħ��������Zn��С������Fe�����ᷴӦʱFe�������䷴Ӧ����ʽΪ��Fe+H2SO4=FeSO4+H2�����������ɵ����������������ʵ�����Ũ�ȣ�
��2��Zn�����ᷴӦʱп��ȫ��Ӧ�����ݷ���ʽZn+H2SO4=ZnSO4+H2������Zn�����ʵ�����������Zn��Fe��������ͬ��
��� �⣺��1��H2SO4��Һ��25mL���ֱ�����������Zn��Fe�����ɵ������������ͬ������Fe��Ħ��������Zn��С������Fe�����ᷴӦʱFe�������䷴Ӧ����ʽΪ��Fe+H2SO4=FeSO4+H2������ͼ���֪���ɵ�����Ϊn��H2��=$\frac{V}{{V}_{m}}$=$\frac{0.224L}{22.4L/mol}$=0.01mol����n��H2SO4��=n��H2��=0.01mol��c��H2SO4��=$\frac{n}{V}$=$\frac{0.01mol}{0.025L}$=0.40mol/L��
�ʴ�Ϊ��0.40��
��2��Zn�����ᷴӦʱп��ȫ��Ӧ����Ӧ����ʽΪZn+H2SO4=ZnSO4+H2������n��Zn��=0.01mol��m��Zn��=nM=0.01mol��65g/mol=0.65g��Zn��Fe��������ͬ������Fe������Ϊ0.65g��
�ʴ�Ϊ��0.65��
���� ���⿼���˸��ݷ���ʽ�ļ��㣬��ȷ����֮�䷢���ķ�Ӧ�ǽⱾ��ؼ����ٽ��ԭ���غ���м��㣬��Ŀ�Ѷ��еȣ�

| A�� | ����ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� | |
| B�� | �ڷ�Һ©�����þƾ���ȡ��ˮ�еĵ⣬��Һʱ�²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| C�� | ����ŨH2SO4��ŨHNO3�Ļ���ʱ��Ӧ��ŨH2SO4�����ӵ�ŨHNO3�У�����ʱ���� | |
| D�� | �������ᾧ�ķ�����ʹNaCl����Һ������ |
| A�� | ����ʱ���þƾ���ֱ�ӽ�ˮ�����Ի�þ��� | |
| B�� | ʵ����ϣ�������ǯȡ����������ʵ�����ϣ��������ִ��� | |
| C�� | ���Լ����ʯ�����Ƭ���沣�������� | |
| D�� | ����������Ӧ�ò��������Ͻ����Է�ֹҺ�ηɽ� |
| A�� | 400ml | B�� | 450ml | C�� | 500ml | D�� | ��ȷ�� |
| A�� | Na+��Fe2+��NO3-��Cl- | B�� | Na+��K+��NO3-��Cl- | ||
| C�� | Na+��K+��Al��OH��4-��Cl- | D�� | NH4+��K+��SO42-��HCO3- |
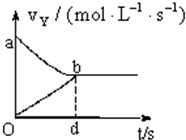 ���ݻ��̶�Ϊ2L���ܱ������У�����X��Y�����2mol���������淴Ӧ��X��g��+2Y��g��?2Z��g��������ƽ�⣬��Y��ʾ�ķ�Ӧ�ٶ�v����v����ʱ��t�Ĺ�ϵ��ͼ��ʾ����Y�ı仯Ũ�ȱ���ʽ��ȷ���ǣ�ʽ��S�Ƕ�Ӧ������������������
���ݻ��̶�Ϊ2L���ܱ������У�����X��Y�����2mol���������淴Ӧ��X��g��+2Y��g��?2Z��g��������ƽ�⣬��Y��ʾ�ķ�Ӧ�ٶ�v����v����ʱ��t�Ĺ�ϵ��ͼ��ʾ����Y�ı仯Ũ�ȱ���ʽ��ȷ���ǣ�ʽ��S�Ƕ�Ӧ������������������| A�� | 2-Saob | B�� | Saob | C�� | Sdob | D�� | 1-Saob |
| A�� | ��12C��ѧ���ʲ�ͬ | B�� | ��C60��Ϊͬ�������� | ||
| C�� | ��12C��Ϊͬλ�� | D�� | ��14N���е���������ͬ |
| A�� | ����ƿ | B�� | ���� | C�� | ��ͷ�ι� | D�� | ��Ͳ |
