��Ŀ����
��10�֣�ij��ɫ����Һ���ܺ����������ӣ�K+��Al3+��Fe3+��Ba2+��NO3����SO42����HCO3����Cl���ȣ�ȡ����Һ��������ʵ�飺
������ɫʯ����ֽ������Һ����ֽ�Ժ�ɫ��
��ȡ��Һ����������ͭƬ��ϡ���Ṳ�ȣ�������ɫ���壬��������������������Ϊ����ɫ��
��ȡ��Һ���������˰�ˮ�а�ɫ�������ɣ��������˹�����ˮ����������ʧ��
��ȡ��Һ�����������Ȼ�����Һ������ɫ������
��ȡʵ�� �� ��ij�����Һ��������������Һ������ɫ�������ټ��˹�����ϡ���ᣬ��������ʧ��

��ش��������⣺
��1����ʵ����У���ͼ��ʾ�IJ�������ȷ����___________________������ţ���
��2����������ʵ���ж�ԭ��Һ�п϶����ڵ�������____________________���϶������ڵ�������_______________��
��3��д����ʵ����йص����ӷ���ʽ��____________________________��
��10�֣� ��1�� B��D ��2�֣�
��2�� NO3-��Al3+��SO42- ��3�֣�
��3�� Fe3+��HCO3-��Ba2+ ��3�֣�
��4�� 3Cu+8H++2NO3-=3Cu2++2NO��+4H2O ��2�֣�
��������

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�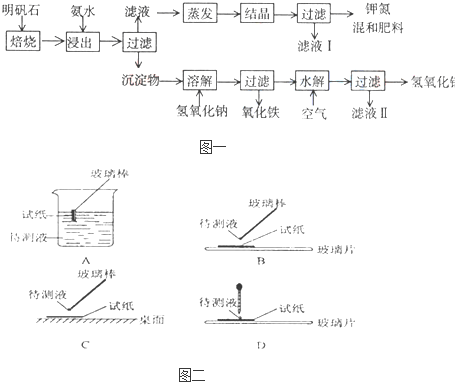
 ij��ɫ����Һ�п��ܺ�Mg2+��Al3+��Fe3+��Cu2+��NH4+��K+�еļ������ӣ�����������Ĺ�������ʱ������ɫ��ζ�����������ͬʱ���ɰ�ɫ����������Ĺ������Ƶ����������������֮��Ĺ�ϵ����ͼ��ʾ���Իش�
ij��ɫ����Һ�п��ܺ�Mg2+��Al3+��Fe3+��Cu2+��NH4+��K+�еļ������ӣ�����������Ĺ�������ʱ������ɫ��ζ�����������ͬʱ���ɰ�ɫ����������Ĺ������Ƶ����������������֮��Ĺ�ϵ����ͼ��ʾ���Իش�
