��Ŀ����
(1)�Ի�ͭ��ұ������ͭ�Ĺ����з�����Ӧ��2Cu2O��Cu2S 6Cu��SO2������Ӧ����������_____________________��
6Cu��SO2������Ӧ����������_____________________��
(2) ��ͭ��������õ��Ĵ�ͭ������Fe��Zn��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ��ڵ�⾫��ͭʱ�����Һ�г�������ͭ�⣬�����ټ���һЩ���ᣬ��������_____________________��
(3)�ھ���ͭ�Ĺ����У����Һ��c(Cu2+)���½���c(Fe2+)��c(Zn2+)�������������趨ʱ��ȥ���е�Fe2+��Zn2+���±�Ϊ�������ʵ��ܶȻ���
ijͬѧ��������³��ӷ�����
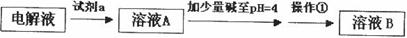
�Լ�a��__________����Ŀ����____________________________________�������ܶȻ��÷����ܹ���ȥ�����ʽ�����������______���������õ��IJ���������____________��
��4����ҵ��ͨ����FeS��ȥ��ˮ�е�Cu2+,д���ó���ת�����ӷ���ʽ
 6Cu��SO2������Ӧ����������_____________________��
6Cu��SO2������Ӧ����������_____________________��(2) ��ͭ��������õ��Ĵ�ͭ������Fe��Zn��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ��ڵ�⾫��ͭʱ�����Һ�г�������ͭ�⣬�����ټ���һЩ���ᣬ��������_____________________��
(3)�ھ���ͭ�Ĺ����У����Һ��c(Cu2+)���½���c(Fe2+)��c(Zn2+)�������������趨ʱ��ȥ���е�Fe2+��Zn2+���±�Ϊ�������ʵ��ܶȻ���
| ���� | 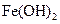 | 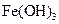 | 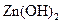 | 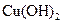 |
�ܶȻ�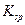 | 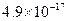 | 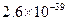 | 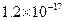 | 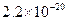 |
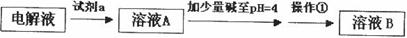
�Լ�a��__________����Ŀ����____________________________________�������ܶȻ��÷����ܹ���ȥ�����ʽ�����������______���������õ��IJ���������____________��
��4����ҵ��ͨ����FeS��ȥ��ˮ�е�Cu2+,д���ó���ת�����ӷ���ʽ
��1��Cu2O��Cu2S,��2�֣�
��2����ǿ���Һ�ĵ����ԣ�����Cu2+��ˮ�⡣��2�֣�
��3��H2O2��1�֣���Fe2+������Fe3+��2�֣� Fe2+��1�֣�©�����ձ�����������2�֣�
��4��Cu2+(aq)+FeS��s����CuS(s)+ Fe2+ (aq)
��2����ǿ���Һ�ĵ����ԣ�����Cu2+��ˮ�⡣��2�֣�
��3��H2O2��1�֣���Fe2+������Fe3+��2�֣� Fe2+��1�֣�©�����ձ�����������2�֣�
��4��Cu2+(aq)+FeS��s����CuS(s)+ Fe2+ (aq)
��

��ϰ��ϵ�д�
�����Ŀ
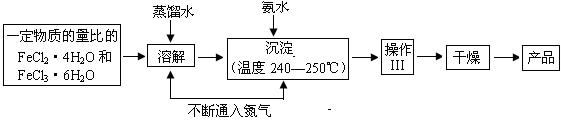
 �������γ������ݣ��������ɵ���������������Χ������ֹ���ij����ۼ����ţ��� �� ��
�������γ������ݣ��������ɵ���������������Χ������ֹ���ij����ۼ����ţ��� �� ��
 �ζ������յ�ʱ����KMnO4��Һ���29.80mL��
�ζ������յ�ʱ����KMnO4��Һ���29.80mL�� ��3I2+3H2O+3K2SO4
��3I2+3H2O+3K2SO4 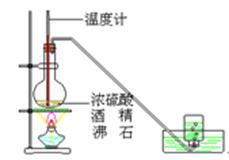
 1)��ͼ����KMnO4��Ũ���ᷴӦ��ȡ���������ļ���װ�á�
1)��ͼ����KMnO4��Ũ���ᷴӦ��ȡ���������ļ���װ�á�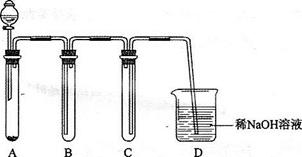

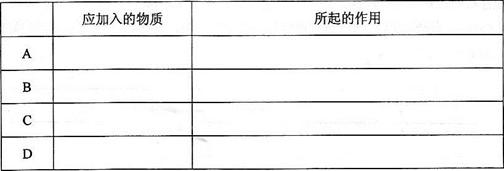
 ��������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��