��Ŀ����
����Ŀ����Ѫ֬����Ӱ�����彡����������E��һ���ٴ����Ƹ�Ѫ֢֬��ҩ�E�ĺϳ�·������(���ַ�Ӧ�������Լ���)��

��֪�� ��CO2��(R1��R2�������)
��CO2��(R1��R2�������)
��ش��������⣺
(1)�Լ����������________���Լ����й����ŵ�������________���ڢڲ��ķ�Ӧ������_____��
(2)�ڢٲ���Ӧ�Ļ�ѧ����ʽ��__________________________��
(3)�ڢ���Ӧ�Ļ�ѧ����ʽ��__________________________��
(4)�ڢ߲���Ӧ�У��Լ���Ϊ�������������ṹ��ʽ��_____________��
(5)C��ͬ���칹��������������ˮ�⣬����X��Y��CH3(CH2)4OH����X�����Ȼ��ͱ�������X��Y�ĺ˴Ź�������ֻ���������͵����շ壬��X��Y�������۷�Ӧ����������Ľṹ��ʽ��____________________��
���𰸡� �״� ��ԭ�� ȡ����Ӧ 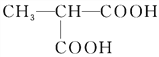 ��2CH3OH
��2CH3OH![]()
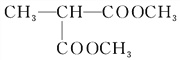 ��2H2O
��2H2O 
![]()
 ��CO2�� CH3I
��CO2�� CH3I 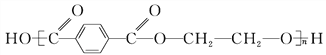
����������1���Լ�I�Ľṹ��ʽΪCH3OH�������Ǽ״����Լ���Ľṹ��ʽΪBrCH2CH2CH2Br�����еĹ�����Ϊ��ԭ�ӣ��Ա�![]() ��
��![]() ��BrCH2CH2CH2Br�Ľṹ��֪����Ӧ������ȡ����Ӧ����2����Ӧ����CH3CH��COOH��2��CH3OH����������Ӧ����CH3CH��COOCH3��2����Ӧ����ʽΪ��CH3CH��COOH��2+2CH3OH
��BrCH2CH2CH2Br�Ľṹ��֪����Ӧ������ȡ����Ӧ����2����Ӧ����CH3CH��COOH��2��CH3OH����������Ӧ����CH3CH��COOCH3��2����Ӧ����ʽΪ��CH3CH��COOH��2+2CH3OH![]() CH3CH��COOCH3��2+2H2O����3����Ӧ���ڼ��������·�������ˮ�ⷴӦ����B��B�ữ����C����CΪ
CH3CH��COOCH3��2+2H2O����3����Ӧ���ڼ��������·�������ˮ�ⷴӦ����B��B�ữ����C����CΪ![]() �������Ϣ��֪D
�������Ϣ��֪D![]() ���ڢ���Ӧ�Ļ�ѧ����ʽ�ǣ�
���ڢ���Ӧ�Ļ�ѧ����ʽ�ǣ�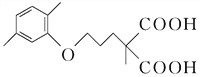
![]()
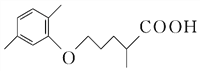
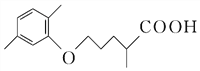 ��CO2������4���ڢ߲���Ӧ�У��Լ���Ϊ������������Ա�D��E�Ľṹ��ʽ��֪�Լ���ΪCH3I����5��C�ķ���ʽΪC15H20O5����ͬ���칹��������������ˮ�⣬˵����������������X��Y��CH3��CH2��4OH��������X�����Ȼ��ͱ�������X��Y�ĺ˴Ź�������ֻ���������͵����շ壬��XΪ
��CO2������4���ڢ߲���Ӧ�У��Լ���Ϊ������������Ա�D��E�Ľṹ��ʽ��֪�Լ���ΪCH3I����5��C�ķ���ʽΪC15H20O5����ͬ���칹��������������ˮ�⣬˵����������������X��Y��CH3��CH2��4OH��������X�����Ȼ��ͱ�������X��Y�ĺ˴Ź�������ֻ���������͵����շ壬��XΪ![]() ��YΪHOCH2CH2OH��X��Y�������۷�Ӧ����������Ľṹ��ʽ�ǣ�
��YΪHOCH2CH2OH��X��Y�������۷�Ӧ����������Ľṹ��ʽ�ǣ�![]() ��
��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�